Effects of Coix Seed Oil on High Fat Diet-Induced Obesity and Dyslipidemia
Abstract
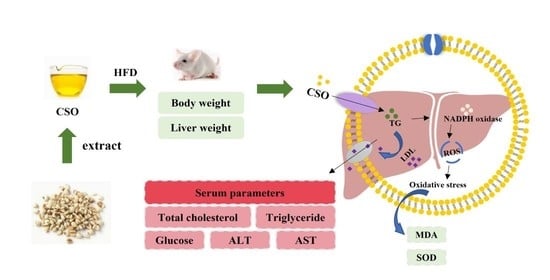
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fatty Acid Composition of Coix Seed Oil
2.3. Experimental Design and Treatment
2.4. Biochemical Assays and Tissue Parameters
2.5. Histopathology
2.6. Isolation of Total Proteins and Western Blotting
2.7. Statistical Evaluation
3. Results
3.1. Fatty Acid Composition and Triacylglycerol Species
3.2. Effects of CSO on Body and Liver Weight
3.3. Effect of CSO on Biochemical Alterations of Serum
3.4. Effect of CSO on Oxidative Stress and Inflammation
3.5. Inflammatory Factors in the Hepatic Tissues
3.6. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bigagli, E.; Cinci, L.; Niccolai, A.; Tredici, M.R.; Biondi, N.; Rodolfi, L.; Lodovici, M.; D’Ambrosio, M.; Mori, G.; Luceri, C. Safety evaluations and lipid-lowering activity of an Arthrospira platensis enriched diet: A 1-month study in rats. Food Res. Int. 2017, 102, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Vega, V.A.; Anzulovich, A.C.; Varas, S.M.; Bonomi, M.R.; Giménez, M.S.; Oliveros, L.B. Effect of nutritional vitamin A deficiency on lipid metabolism in the rat heart: Its relation to PPAR gene expression. Nutrition 2009, 25, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Orešič, M. Metabolomics, a novel tool for studies of nutrition, metabolism and lipid dysfunction. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Miles, E.A.; Calder, P.C. A review of the potential health benefits of pine nut oil and its characteristic fatty acid pinolenic acid. J. Funct. Foods 2016, 23, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, T.; Rasmussen, A.; Yang, L.; Kaul, C.; Black, S.; Galbiati, H.; Conway, S.J.; Miller, S.; Blount, P.; Booth, I.R. Interaction of the Mechanosensitive Channel, MscS, with the Membrane Bilayer through Lipid Intercalation into Grooves and Pockets. J. Mol. Biol. 2019, 431, 3339–3352. [Google Scholar] [CrossRef]
- Perona, J.S. Membrane lipid alterations in the metabolic syndrome and the role of dietary oils. Biochim. Biophys. Acta 2017, 1859, 1690–1703. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Zahradka, P.; Neumann, S.; Aukema, H.M.; Taylor, C.G. Adipocyte lipid storage and adipokine production are modulated by lipoxygenase-derived oxylipins generated from 18-carbon fatty acids. Int. J. Biochem. Cell Biol. 2017, 88, 23–30. [Google Scholar] [CrossRef]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef]
- Jones, J.R.; Barrick, C.; Kim, K.A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPAR in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beermann, C.; Neumann, S.; Fussbroich, D.; Zielen, S.; Schubert, R. Combinations of distinct long-chain polyunsaturated fatty acid species for improved dietary treatment against allergic bronchial asthma. Nutrition 2016, 32, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Sakaguchi, S. Regulatory T Cells Expressing PPAR-γ Control Inflammation in Obesity. Cell Metab. 2012, 16, 4–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, A.J.; Zhao, S.; Liang, H.; Qiu, T.Q.; Chen, G. Ultrasound assisted supercritical fluid extraction of oil and coixenolide from adlay seed. Ultrason. Sonochem. 2007, 14, 219–224. [Google Scholar] [CrossRef]
- Yu, F.; Gao, J.; Zeng, Y.; Liu, C.X. Inhibition of Coix seed extract on fatty acid synthase, a novel target for anticancer activity. J. Ethnopharmacol. 2008, 119, 252–258. [Google Scholar] [CrossRef]
- Mei, Y.H.; Nan, W.S.; Ping, N.S.; Yong, X.M. Coix polysaccharides: Gut microbiota regulation and immunomodulatory. Bioact. Carbohydr. Diet. Fibre 2018, 16, 53–61. [Google Scholar] [CrossRef]
- Yang, H.; Min, W.; Bi, P.; Zhou, H.; Huang, F. Stimulatory effects of Coix lacryma-jobi oil on the mycelial growth and metabolites biosynthesis by the submerged culture of Ganoderma lucidum. Biochem. Eng. J. 2013, 76, 77–82. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Su, A.; Zhang, Y.; Yuan, J.; Ju, X. Structural characterization of phenolic compounds and antioxidant activity of the phenolic-rich fraction from defatted adlay seed meal. Food Chem. 2016, 196, 509–517. [Google Scholar] [CrossRef]
- Rao, P.N.; Nirmala, A. Chromosomal basis of evolution in the genus Coix L. (Maydeae): A critical appraisal. Nucleus 2011, 53, 13–24. [Google Scholar] [CrossRef]
- Wu, T.; Charles, A.; Huang, T. Determination of the contents of the main biochemical compounds of Adlay. Food Chem. 2007, 104, 1509–1515. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, X.; Ma, X.; Xiong, H.; Zeng, Z.; Peng, H.; Hu, J. Enzymatic production of trans-free shortening from coix seed oil, fully hydrogenated palm oil and Cinnamomum camphora seed oil. Food Biosci. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Yang, Z.; Wen, A.; Qin, L.; Zhu, Y. Effect of Coix Seed Extracts on Growth and Metabolism of Limosilactobacillus reuteri. Foods 2022, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-C.; Chen, H.-H.; Chiang, W. Adlay (薏苡 yì yĭ; “soft-shelled job’s tears”; the seeds of Coix lachryma-jobi L. var. ma-yuen Stapf) is a Potential Cancer Chemopreventive Agent toward Multistage Carcinogenesis Processes. J. Tradit. Complement. Med. 2012, 2, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, Y.; Lu, S.; Sun, X.; Yin, Y.; Wang, K.; Liu, S. Coix seed oil regulates mitochondrial functional damage to induce apoptosis of human pancreatic cancer cells via the PTEN/PI3K/AKT signaling pathway. Mol. Biol. Rep. 2022, 49, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Tong, X.; Lu, F.; Mao, W.; Fu, L.; Deng, L.; Liu, X.; Li, C.; Zhang, L.; et al. The effect of coix seed on the nutritional status of peritoneal dialysis patients: A pilot study. Complement. Med. 2014, 22, 40–48. [Google Scholar] [CrossRef]
- Son, B.K.; Kim, J.Y.; Lee, S.S. Effect of adlay, buckwheat and barley on lipid metabolism and aorta histopathology in rats fed an obesogenic diet. Ann. Nutr. Metab. 2008, 52, 181–187. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, Z.; Zheng, J.; Wang, Y.; Chen, Q.; Liu, R.; Liu, X.; Zhang, S. Optimizations and comparison of two supercritical extractions of adlay oil. Innov. Food Sci. Emerg. Technol. 2012, 13, 128–133. [Google Scholar] [CrossRef]
- Chung, C.P.; Hsia, S.M.; Lee, M.Y.; Chen, H.J.; Cheng, F.; Chan, L.C.; Kuo, Y.H.; Lin, Y.L.; Chiang, W. Gastroprotective activities of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on the growth of the stomach cancer AGS cell line and indomethacin-induced gastric ulcers. J. Agric. Food Chem. 2011, 59, 6025–6033. [Google Scholar] [CrossRef]
- Sun, B.; Luo, C.; Cui, W.; Sun, J.; He, Z. Chemotherapy agent-unsaturated fatty acid prodrugs and prodrug-nanoplatforms for cancer chemotherapy. J. Control. Release 2017, 264, 145–159. [Google Scholar] [CrossRef]
- Chen, X.Y.; Liao, D.C.; Yu, Y.T.; Wei, C.M.; Xuan, L.Y.; Li, S.; Wang, H.B. Coix seed oil prolongs lifespan and enhances stress resistance in Caenorhabditis elegans. Biogerontology 2020, 21, 245–256. [Google Scholar] [CrossRef]
- Chiang, H.; Lu, H.-F.; Chen, J.-C.; Chen, Y.-H.; Sun, H.-T.; Huang, H.-C.; Tien, H.-H.; Huang, C.; Martel, F. Adlay Seed (Coix lacryma-jobi L.) Extracts Exhibit a Prophylactic Effect on Diet-Induced Metabolic Dysfunction and Nonalcoholic Fatty Liver Disease in Mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Fan, Z.-Y.; Wang, H.-Y.; Wen, D.-C.; Zhang, S.-Y. Effect of polysaccharides from adlay seed on anti-diabetic and gut microbiota. Food Funct. 2019, 10, 4372–4380. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Jiang, B.-K.; Zheng, W.-H.; Zhang, S.-Y.; Li, J.-J.; Fan, Z.-Y. Preparation, characterization and anti-diabetic activity of polysaccharides from adlay seed. Int. J. Biol. Macromol. 2019, 139, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wei, W.; Su, H.; Zou, X.; Wang, X. Evaluation of sn-2 fatty acid composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. Food Chem. 2018, 242, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.A.; Heo, Y.J.; Lee, K.T. Physicochemical characteristics of fat blend from hydrogenated coconut oil and acyl migrated palm mid-fraction. Food Chem. 2019, 275, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jeon, Y.E.; Jung, J.I.; Kim, S.M.; Hong, S.H.; Lee, J.; Hwang, J.S.; Hwang, M.O.; Kwon, K.; Kim, E.J. Anti-obesity effect of Cydonia oblonga Miller extract in high-fat diet-induced obese C57BL/6 mice. J. Funct. Foods 2022, 89, 104945. [Google Scholar] [CrossRef]
- Park, J.-H.; Ahn, E.-K.; Ko, H.-J.; Hwang, M.H.; Cho, Y.-R.; Lee, D.-R.; Choi, B.-K.; Seo, D.-W.; Oh, J.S. Spiraea prunifolia leaves extract inhibits adipogenesis and lipogenesis by promoting β-oxidation in high fat diet-induced obese mice. Biomed. Pharmacother. 2022, 149, 112889. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, Z.; Zhao, Y.; Kang, C.; Zhang, X.; Tian, Y.; Yu, J.; Cao, H.; Wang, M. The anti-diabetic activity of polyphenols-rich vinegar extract in mice via regulating gut microbiota and liver inflammation. Food Chem. 2022, 393, 133443. [Google Scholar] [CrossRef]
- Zhao, C.; Fan, J.; Liu, Y.; Guo, W.; Cao, H.; Xiao, J.; Wang, Y.; Liu, B. Hepatoprotective activity of Ganoderma lucidum triterpenoids in alcohol-induced liver injury in mice, an iTRAQ-based proteomic analysis. Food Chem. 2019, 271, 148–156. [Google Scholar] [CrossRef]
- Donado-Pestana, C.M.; Pessoa, E.V.M.; Rodrigues, L.; Rossi, R.; Moura, M.H.C.; Dos Santos-Donado, P.R.; Castro, E.; Festuccia, W.T.; Genovese, M.I. Polyphenols of cambuci (Campomanesia phaea (O. Berg.)) fruit ameliorate insulin resistance and hepatic steatosis in obese mice. Food Chem. 2021, 340, 128169. [Google Scholar] [CrossRef]
- Bracco, U. Effect of triglyceride structure on fat absorption. Am. J. Clin. Nutr. 1994, 60, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Moens, L.G.; Carrillo, C.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Kinetic approach to study the relation between in vitro lipid digestion and carotenoid bioaccessibility in emulsions with different oil unsaturation degree. J. Funct. Foods 2018, 41, 135–147. [Google Scholar] [CrossRef]
- Ramırez, M.; Amate, L.; Gil, A. Absorption and distribution of dietary fatty acids from different sources. Early Hum. Dev. 2001, 65, S95–S101. [Google Scholar] [CrossRef]
- Foresti, M.L.; Ferreira, M.L. Lipase-catalyzed acidolysis of tripalmitin with capric acid in organic solvent medium: Analysis of the effect of experimental conditions through factorial design and analysis of multiple responses. Enzym. Microb. Technol. 2010, 46, 419–429. [Google Scholar] [CrossRef]
- Dasilva, G.; Medina, I. Lipidomic methodologies for biomarkers of chronic inflammation in nutritional research: Omega-3 and omega-6 lipid mediators. Free. Radic. Biol. Med. 2019, 144, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Pimienta, M.; Seki, E. Alcoholic liver disease: A current molecular and clinical perspective. Liver Res. 2018, 2, 161–172. [Google Scholar] [CrossRef]
- Ide, T.; Iwase, H.; Amano, S.; Sunahara, S.; Tachihara, A.; Yagi, M.; Watanabe, T. Physiological effects of gamma-linolenic acid and sesamin on hepatic fatty acid synthesis and oxidation. J. Nutr. Biochem. 2017, 41, 42–55. [Google Scholar] [CrossRef]
- Bogie, J.F.J.; Haidar, M.; Kooij, G.; Hendriks, J.J.A. Fatty acid metabolism in the progression and resolution of CNS disorders. Adv. Drug Deliv. Rev. 2020, 159, 198–213. [Google Scholar] [CrossRef]
- Mohammadian, M.; Mianabadi, M.; Zargari, M.; Karimpour, A.; Khalafi, M.; Amiri, F.T. Effects of Olive Oil supplementation on Sodium Arsenate-induced Hepatotoxicity in Mice. Int. J. Prev. Med. 2018, 9, 59. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Xia, Y.; Xu, J.; Li, Q.; Zhang, C.; Li, M. Effects of phytochemicals from plant-based functional foods on hyperlipidemia and their underpinning mechanisms. Trends Food Sci. Technol. 2020, 103, 304–320. [Google Scholar] [CrossRef]
- Zhu, F. Coix: Chemical composition and health effects. Trends Food Sci. Technol. 2017, 61, 160–175. [Google Scholar] [CrossRef]





| Peanut Oil (PE) | Lard (LA) | Coix Seed Oil (CSO) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid | Total Content (%) | sn-2 | sn-3 | Total Content (%) | sn-2 | sn-3 | Total Content (%) | sn-2 | sn-3 |
| C8:0 | 0.41 | 0.03 | 0.38 | 0.21 | 0.17 | 0.04 | 3.41 | 0.43 | 2.98 |
| C10:0 | 6.28 | 3.51 | 2.77 | 4.28 | 2.15 | 2.13 | 45.28 * | 24.5 | 20.78 |
| C12:0 | 10.21 | 8.24 | 1.97 | 8.71 | 6.34 | 2.37 | 42.21 * | 22.24 | 19.97 |
| C14:0 | 21.22 * | 12.23 | 8.99 | 12.62 | 10.45 | 2.17 | 2.22 | 1.23 | 0.99 |
| C16:0 | 28.03 | 17.55 | 10.48 | 30.03 * | 11.45 | 18.58 | 1.03 | 0.45 | 0.58 |
| C18:0 | 31.67 * | 16.32 | 15.35 | 38.67 * | 19.32 | 19.35 | 0.67 | 0.32 | 0.35 |
| 9C18:1 | 0.63 | 0.32 | 0.31 | 0.91 | 0.32 | 0.59 | 3.21 | 1.32 | 1.89 |
| 11C18:1 | 0.23 | 0.12 | 0.11 | 1.13 | 1.02 | 0.11 | 0.23 | 0.02 | 0.21 |
| 9c12cC18:2 | 0.44 | 0.12 | 0.32 | 0.58 | 0.32 | 0.26 | 1.48 | 0.82 | 0.66 |
| 9t12c15tC18:3 | 0.91 | 0.43 | 0.48 | 0.92 | 0.33 | 0.59 | 0.93 | 0.53 | 0.4 |
| 9c12c15tC18:3 | 0.33 | 0.15 | 0.18 | 0.76 | 0.15 | 0.61 | 1.13 | 0.93 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Xue, S.; Dai, B.; Zhao, H. Effects of Coix Seed Oil on High Fat Diet-Induced Obesity and Dyslipidemia. Foods 2022, 11, 3267. https://doi.org/10.3390/foods11203267
Chen L, Xue S, Dai B, Zhao H. Effects of Coix Seed Oil on High Fat Diet-Induced Obesity and Dyslipidemia. Foods. 2022; 11(20):3267. https://doi.org/10.3390/foods11203267
Chicago/Turabian StyleChen, Lichun, Songwen Xue, Binhao Dai, and Huimin Zhao. 2022. "Effects of Coix Seed Oil on High Fat Diet-Induced Obesity and Dyslipidemia" Foods 11, no. 20: 3267. https://doi.org/10.3390/foods11203267