The Effect of Microwave Irradiation on the Representation and Growth of Moulds in Nuts and Almonds
Abstract
:1. Introduction
2. Materials and Methods
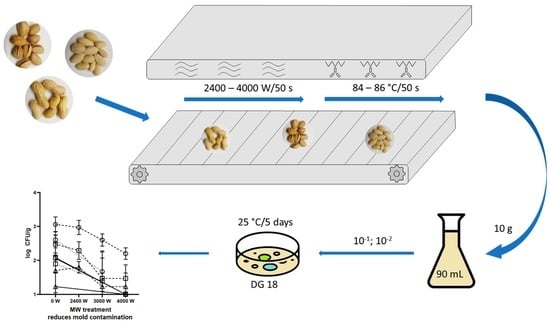
2.1. Analysed Samples
2.2. Treatment Conditions
2.3. Microbial Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holland, B.; Unwin, I.D.; Buss, D. Fruits and Nuts: The Composition of Foods, 5th ed.; The Royal Society of Chemistry: Cambridge, UK, 1992; pp. 87–104. ISBN 0851863868. [Google Scholar]
- Ros, E. Nuts: Health effects. In Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 111–118. [Google Scholar]
- Basaran, P.; Akhan, Ü. Microwave irradiation of hazelnuts for the control of aflatoxin producing Aspergillus parasiticus. Innov. Food Sci. Emerg. Technol. 2010, 11, 113–117. [Google Scholar] [CrossRef]
- da Silva, A.C.; Sarturi, H.J.; Dall’Oglio, E.L.; Soares, M.A.; de Sousa, P.T.; Gomes de Vasconcelos, L.; Kuhnen, C.A. Microwave drying and disinfestation of Brazil nut seeds. Food Control 2016, 70, 119–129. [Google Scholar] [CrossRef]
- Chou, S.K.; Chua, K.J. New hybrid drying technologies for heat sensitive foodstuffs. Trends Food Sci. Technol. 2001, 12, 359–369. [Google Scholar] [CrossRef]
- Guzik, P.; Kulawik, P.; Zając, M.; Migdał, W. Microwave applications in the food industry: An overview of recent developments. Crit. Rev. Food Sci. Nutr. 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.A.; Norton, T.; Alagusundaram, K.; Tiwari, B.K. Novel drying techniques for the food industry. Food Eng. Rev. 2014, 6, 43–55. [Google Scholar] [CrossRef]
- Kumar, Y. Application of microwave in food drying. Int. J. Eng. Stud. Tech. Approach 2015, 1, 9–24. [Google Scholar]
- Poltronieri, P.; Santino, A.; Ciarmiello, L.F.; Hubert, J. Application of microwave and RF in food processing, microorganisms and pest control. Mediterr. Microw. Symp. 2015, 2015, 1–4. [Google Scholar]
- Guo, Q.; Sun, D.-W.; Cheng, J.-H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Meda, V.; Orsat, V.; Raghavan, V. Microwave heating and the dielectric properties of foods. In The Microwave Processing of Foods, 2nd ed.; Regier, M., Knoerzer, K., Schubert, H., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 23–43. [Google Scholar]
- Kouchakzadeh, A.; Shafeei, S. Modeling of microwave–convective drying of pistachios. Energy Convers. Manag. 2010, 51, 2012–2015. [Google Scholar] [CrossRef]
- Cebrián, G.; Condón, S.; Mañas, P. Physiology of the inactivation of vegetative bacteria by thermal treatments: Mode of action, influence of environmental factors and inactivation kinetics. Foods 2017, 6, e107. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Grundler, W.; Keilmann, F. Sharp resonances in yeast growth prove nonthermal sensitivity to microwaves. Phys. Rev. Lett. 1983, 51, 1214–1216. [Google Scholar] [CrossRef]
- Dudley, G.B.; Richert, R.; Stiegman, A.E. On the existence of and mechanism for microwave-specific reaction rate enhancement. Chem. Sci. 2015, 6, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Knoerzer, K.; Regier, M.; Erle, U.; Pardey, K.K.; Schubert, H. Development of a model food for microwave processing and the prediction of its physical properties. J. Microw. Power Electromagn. Energy 2016, 39, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.N.; Anand, T.; Sharma, M.; Gupta, R.K. Microwave technology for disinfestation of cereals and pulses: An overview. J. Food Sci. Technol. 2014, 51, 3568–3576. [Google Scholar] [CrossRef] [Green Version]
- Ekezie, F.-G.C.; Sun, D.-W.; Han, Z.; Cheng, J.-H. Microwave–assisted food processing technologies for enhancing product quality and process efficiency: A review of recent developments. Trends Food Sci. Technol. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- Hamoud–Agha, M.M.; Curet, S.; Simonin, H.; Boillereaux, L. Holding time effect on microwave inactivation of Escherichia coli K12: Experimental and numerical investigations. J. Food Eng. 2014, 143, 102–113. [Google Scholar] [CrossRef]
- Deliu, I.; Giosanu, D.; Stănescu, C. The microwaves effects on liquid foods. Sci. Bull. Ser. F. Biotechnol. 2013, XVII, 208–211. [Google Scholar]
- Dańczuk, M.; Łomotowski, J. Appliction of microwave energy to the hygienization of sewage sludge. Environ. Prot. Eng. 2010, 36, 77–86. [Google Scholar]
- Bozkut–Cekmer, H.; Davidson, P.M. Microwaves for microbial inactivation–efficiency and inactivation kinetics. In The Microwave Processing of Foods; Woodhead Publishing: Duxford, UK, 2017; pp. 220–251. ISBN 9780081005286. [Google Scholar]
- Zhang, X.; Liu, X.; Karim, R.; Ismail, M.H.S. Preparation and microwave preservation of wheat rice blending wet noodle. J. Food Processing Preserv. 2018, 42, e13362. [Google Scholar] [CrossRef]
- Pucciarelli, A.B.; Benassi, F.O. Inactivation of Salmonella enteritidis on raw poultry using microwave heating. Braz. Arch. Biol. Technol. 2005, 48, 939–945. [Google Scholar] [CrossRef]
- Bauza–Kaszewska, J.; Skowron, K.; Paluszak, Z.; Dobrzański, Z.; Śrutek, M. Effect of microwave radiation on microorganisms in fish meals. Ann. Anim. Sci. 2014, 14, 623–636. [Google Scholar] [CrossRef] [Green Version]
- Lakins, D.G.; Echeverry, A.; Alvarado, C.Z.; Brooks, J.C.; Brashears, M.T.; Brashears, M.M. Quality of and mold growth on white enriched bread for military rations following directional microwave treatment. J. Food Sci. 2008, 73, M99–M103. [Google Scholar] [CrossRef]
- Reddy, M.V.B.; Raghavan, G.S.V.; Kushalappa, A.C.; Paulitz, T.C. Effect of microwave treatment on quality of wheat seeds infected with Fusarium graminearum. J. Agric. Eng. Res. 1998, 71, 113–117. [Google Scholar] [CrossRef]
- ISO 21527–2:2008; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95. ISO: Geneve, Switzerland, 2008.
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008; p. 771. ISBN 9780851998268. [Google Scholar]
- Datta, A.K.; Davidson, P.M. Microwave and radio frequency processing. J. Food Sci. 2000, 65, 32–41. [Google Scholar] [CrossRef]
- Regier, M.; Knoerzer, K.; Schubert, H. Index. In The Microwave Processing of Foods, 2nd ed.; Woodhead Publishing: Duxford, UK, 2017; p. 448. ISBN 9780081005286. [Google Scholar]
- Schlimbach, J.; Ogale, A. Out–of–autoclave curing process in polymer matrix composites. In Manufacturing Techniques for Polymer Matrix Composites (PMCs); Advani, S.G., Hsiao, K.-T., Eds.; Woodhead Publishing: Duxford, UK, 2012; pp. 435–480. ISBN 9780857090676. [Google Scholar]
- Hamid, M.; Thomas, T.; El–Saba, A.; Stapleton, W.; Sakla, A.; Rahman, A.; Byrne, P.; VanLandingham, D.; McCombs, C. The effects of microwaves on airborne microorganisms. J. Microw. Power Electromagn. Energy 2016, 36, 37–45. [Google Scholar] [CrossRef]
- Knox, O.G.G.; McHugh, M.J.; Fountaine, J.M.; Havis, N.D. Effects of microwaves on fungal pathogens of wheat seed. Crop Prot. 2013, 50, 12–16. [Google Scholar] [CrossRef]
- Cavalcante, M.J.B.; Muchovej, J.J. Microwave irradiation of seeds and selected fungal spores. Seed Sci. Technol. 1993, 21, 247–253. [Google Scholar]
- Chen, H.L. Microwave radiation decontamination of mildew infected cotton. Text. Res. J. 2001, 71, 247–254. [Google Scholar] [CrossRef]
- Patil, H.; Shah, N.G.; Hajare, S.N.; Gautam, S.; Kumar, G. Combination of microwave and gamma irradiation for reduction of aflatoxin B-1 and microbiological contamination in peanuts (Arachis hypogaea L.). World Mycotoxin J. 2019, 12, 269–280. [Google Scholar] [CrossRef]
- Dababneh, B.F. An innovative microwave process for microbial decontamination of spices and herbs. Afr. J. Microbiol. Res. 2013, 7, 636–645. [Google Scholar]

| Sample | Treatment Performance (W) | Mould Count (log CFU/g) | ||
|---|---|---|---|---|
| After Treatment | After 3 Months | After 6 Months | ||
| almonds | 0 | 2.70 ± 0.19 b | 2.98 ± 0.12 d | 2.74 ± 0.05 b |
| almonds | 2400 | 2.60 ± 0.18 b | 2.70 ± 0.06 c | 2.78 ± 0.09 b |
| almonds | 3000 | 2.18 ± 0.11 a | 2.18 ± 0.13 b | 2.48 ± 0.14 a |
| almonds | 4000 | 2.08 ± 0.12 a | 1.70 ± 0.25 a | 2.40 ± 0.18 a |
| peanuts | 0 | 2.08 ± 0.09 b | 2.18 ± 0.12 b | 2.10 ± 0.14 b |
| peanuts | 4000 | ND | ND a | ND |
| pistachios | 0 | 2.83 ± 0.11 b | 2.00 ± 0.05 b | 2.18 ± 0.10 b |
| pistachios | 3000 | 1.88 ± 0.16 a | 1.70 ± 0.25 a | 1.60 ± 0.08 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popelářová, E.; Vlková, E.; Švejstil, R.; Kouřimská, L. The Effect of Microwave Irradiation on the Representation and Growth of Moulds in Nuts and Almonds. Foods 2022, 11, 221. https://doi.org/10.3390/foods11020221
Popelářová E, Vlková E, Švejstil R, Kouřimská L. The Effect of Microwave Irradiation on the Representation and Growth of Moulds in Nuts and Almonds. Foods. 2022; 11(2):221. https://doi.org/10.3390/foods11020221
Chicago/Turabian StylePopelářová, Eva, Eva Vlková, Roman Švejstil, and Lenka Kouřimská. 2022. "The Effect of Microwave Irradiation on the Representation and Growth of Moulds in Nuts and Almonds" Foods 11, no. 2: 221. https://doi.org/10.3390/foods11020221