Enhancement of the Antioxidant Capacity of Thyme and Chestnut Honey by Addition of Bee Products
Abstract
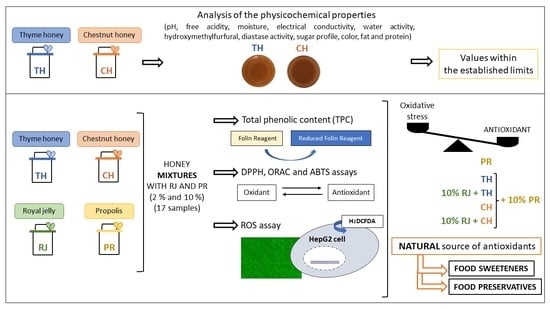
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Cell Culture
2.3. Chemicals and Reagents
2.4. Analysis of the Physicochemical Properties of Thyme and Chestnut Honey Samples
2.4.1. Sugar Profile
2.4.2. Color Determination
2.4.3. Determination of Fat and Protein
2.5. Total Phenolic Compounds (TPC)
2.6. Antioxidant Capacity
2.6.1. Determination of Radical Scavenging Capacity against DPPH
2.6.2. Determination of the Oxygen Radical Absorbance Capacity (ORAC)
2.6.3. ABTS Scavenging Assay
2.7. Reactive Oxygen Species (ROS) Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Thyme and Chestnut Honey
3.2. Total Phenolic Compounds (TPC) of Spanish Thyme and Chestnut Honey and Their Mixtures with Royal Jelly and Propolis
3.3. Antioxidant Capacity of Spanish Thyme and Chestnut Honey and Their Mixtures with Royal Jelly and Propolis
3.4. Time Course of ROS Production induced by Spanish Thyme and Chestnut Honey and Their Mixtures with Royal Jelly and Propolis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2). Phytother. Res. 2021, 35, 743–750. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey Bee Products: Preclinical and Clinical Studies of Their Anti-inflammatory and Immunomodulatory Properties. Front. Nutr. 2022, 8, 761267. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.I.; Boer, J.C.; Wilson, K.L.; Plebanski, M.; Mohamud, R.; Mustafa, M.Z. Antioxidant-Based Medicinal Properties of Stingless Bee Products: Recent Progress and Future Directions. Biomolecules 2020, 10, 923. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Plutino, M.; Lucini, L.; Aromolo, R.; Martinelli, E.; Souto, E.B.; Santini, A.; Pignatti, G. Bee Products: A Representation of Biodiversity, Sustainability, and Health. Life 2021, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- El Menyiy, N.; Akdad, M.; Elamine, Y.; Lyoussi, B. Microbiological Quality, Physicochemical Properties, and Antioxidant Capacity of Honey Samples Commercialized in the Moroccan Errachidia Region. J. Food Qual. 2020, 2020, 7383018. [Google Scholar] [CrossRef]
- Masad, R.J.; Haneefa, S.M.; Mohamed, Y.A.; Al-Sbiei, A.; Bashir, G.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. The Immunomodulatory Effects of Honey and Associated Flavonoids in Cancer. Nutrients 2021, 13, 1269. [Google Scholar] [CrossRef]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey Volatiles as a Fingerprint for Botanical Origin—A Review on their Occurrence on Monofloral Honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, S.; Foster, K.; Lim, L.Y.; Hammer, K.; Locher, C. A Review of the Phytochemistry and Bioactivity of Clover Honeys (Trifolium spp.). Foods 2022, 11, 1901. [Google Scholar] [CrossRef]
- Martinez-Armenta, C.; Camacho-Rea, M.C.; Martínez-Nava, G.A.; Espinosa-Velázquez, R.; Pineda, C.; Gomez-Quiroz, L.E.; López-Reyes, A. Therapeutic Potential of Bioactive Compounds in Honey for Treating Osteoarthritis. Front. Pharmacol. 2021, 12, 642836. [Google Scholar] [CrossRef] [PubMed]
- Aryappalli, P.; Shabbiri, K.; Masad, R.J.; Al-Marri, R.H.; Haneefa, S.M.; Mohamed, Y.A.; Arafat, K.; Attoub, S.; Cabral-Marques, O.; Ramadi, K.B.; et al. Inhibition of Tyrosine-Phosphorylated STAT3 in Human Breast and Lung Cancer Cells by Manuka Honey is Mediated by Selective Antagonism of the IL-6 Receptor. Int. J. Mol. Sci. 2019, 20, 4340. [Google Scholar] [CrossRef]
- Jenkins, R.; Burton, N.; Cooper, R. Proteomic and genomic analysis of methicillin-resistant Staphylococcus aureus (MRSA) exposed to manuka honey In vitro demonstrated down-regulation of virulence markers. J. Antimicrob. Chemother. 2014, 69, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.; Sulaiman, S.; Boukraa, L. Antioxidant properties of honey and its role in preventing health disorder. Open Nutraceuticals J. 2010, 3, 6–16. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef] [Green Version]
- González-Porto, A.V.; Martín Arroyo, T.; Bartolomé Esteban, C. How soil type (gypsum or limestone) influences the properties and composition of thyme honey. Springerplus 2016, 5, 1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escriche, I.; Sobrino-Gregorio, L.; Conchado, A.; Juan-Borrás, M. Volatile profile in the accurate labelling of monofloral honey. The case of lavender and thyme honey. Food Chem. 2017, 226, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koca, I.; Tekguler, B.; Turkyilmaz, B.; Tasci, B. Some physical, chemical and antioxidant properties of chestnut (Castanea sativa Mill.) honey produced in Turkey. Acta Hortic. 2018, 1220, 227–234. [Google Scholar] [CrossRef]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [Green Version]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Ebiloma, G.U.; Ichoron, N.; Siheri, W.; Watson, D.G.; Igoli, J.O.; De Koning, H.P. The Strong Anti-Kinetoplastid Properties of Bee Propolis: Composition and Identification of the Active Agents and Their Biochemical Targets. Molecules 2020, 25, 5155. [Google Scholar] [CrossRef] [PubMed]
- Assumpção, J.H.M.; Takeda, A.A.S.; Sforcin, J.M.; Rainho, C.A. Effects of Propolis and Phenolic Acids on Triple-Negative Breast Cancer Cell Lines: Potential Involvement of Epigenetic Mechanisms. Molecules 2020, 25, 1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boufadi, M.Y.; Soubhye, J.; Van Antwerpen, P. Anti-inflammatory, antioxidant effects, and bioaccessibility of Tigzirt propolis. J. Food Biochem. 2021, 45, e13663. [Google Scholar] [CrossRef]
- Hozzein, W.N.; Badr, G.; Al Ghamdi, A.A.; Sayed, A.; Al-Waili, N.S.; Garraud, O. Topical application of propolis enhances cutaneous wound healing by promoting TGF-beta/Smad-mediated collagen production in a streptozotocin-induced type I diabetic mouse model. Cell. Physiol. Biochem. 2015, 37, 940–954. [Google Scholar] [CrossRef]
- Segueni, N.; Keskin, Ş.; Kadour, B.; Kolaylı, S.; Salah, A. Comparison between Phenolic Content, Antioxidant, and Antibacterial Activity of Algerian and Turkish Propolis. Comb. Chem. High Throughput Screen. 2021, 24, 1679–1687. [Google Scholar] [CrossRef]
- Hotta, S.; Uchiyama, S.; Ichihara, K. Brazilian red propolis extract enhances expression of antioxidant enzyme genes In vitro and in vivo. Biosci. Biotechnol. Biochem. 2020, 84, 1820–1830. [Google Scholar] [CrossRef]
- Kunugi, H.; Mohammed Ali, A. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslan, Z.; Aksoy, L. Anti-inflammatory effects of royal jelly on ethylene glycol induced renal inflammation in rats. Int. Braz. J. Urol. 2015, 41, 1008–1013. [Google Scholar] [CrossRef] [Green Version]
- Al-Kushi, A.G.; Header, E.A.; ElSawy, N.A.; Moustafa, R.A.; Alfky, N.A.A. Antioxidant effect of royal jelly on immune status of hyperglycemic rats. Pharmacogn. Mag. 2018, 14, 528. [Google Scholar] [CrossRef]
- Nakaya, M.; Onda, H.; Sasaki, K.; Yukiyoshi, A.; Tachibana, H.; Yamada, K. Effect of royal jelly on bisphenol A-induced proliferation of human breast cancer cells. Biosci. Biotechnol. Biochem. 2007, 71, 253–255. [Google Scholar] [CrossRef]
- Mostafa, R.E.; El-Marasy, S.A.; Abdel Jaleel, G.A.; Bakeer, R.M. Protective effect of royal jelly against diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats. Heliyon 2020, 6, e03330. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, M.; Lin, T.; Wang, L.; Wang, G.; Chen, T.; Su, S. Royal jelly from different floral sources possesses distinct wound-healing mechanisms and ingredient profiles. Food Funct. 2021, 12, 12059–12076. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niaz, K.; Shah, M.A.; Khan, F.; Saleem, U.; Vargas, C.; Panichayupakaranant, P. Bioavailability and safety of phytonutrients. In Phytonutrients in Food; Nabavi, S.M., Suntar, I., Barreca, D., Khan, H., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 117–136. [Google Scholar]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [Green Version]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Wilczyńska, A. Effect of filtration on colour, antioxidant activity and total phenolics of honey. Food Sci. Technol. 2014, 57, 767–774. [Google Scholar] [CrossRef]
- Ullah, H.; Hussain, Y.; Santarcangelo, C.; Baldi, A.; Di Minno, A.; Khan, H.; Xiao, J.; Daglia, M. Natural Polyphenols for the Preservation of Meat and Dairy Products. Molecules 2022, 27, 1906. [Google Scholar] [CrossRef]
- Anand, S.P.; Sati, N. Artificial preservatives and their harmful effects: Looking toward nature for safer alternatives. Int. J. Pharm. Sci. Res. 2013, 4, 2496. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Elsadek, M.F.; Mohamed, A.S.; Taha, A.E.; Ahmed, B.M.; Saad, A.M. Effects of Chemical and Natural Additives on Cucumber Juice’s Quality, Shelf Life, and Safety. Foods 2020, 9, 639. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of Phenolic Compounds for Food Preservation: Food Additive and Active Packaging. In Phenolic Compounds–Biological Activity; Soto-Hernández, M., Palma-Tenango, M., García-Mateos, R., Eds.; IntechOpen: London, UK, 2017; pp. 39–58. [Google Scholar]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Haza, A.I. Antiproliferative and apoptotic effects of spanish honeys. Pharmacogn. Mag. 2013, 9, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haza, A.I.; Morales, P. Spanish honeys protect against food mutagen-induced DNA damage. J. Sci. Food Agric. 2013, 93, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Von Der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- International Honey Commission. Harmonized Methods of the International Honey Commission. 2009. Available online: http://www.beehexagon.net/en/network.htm (accessed on 1 April 2021).
- González-Ceballos, L.; Fernández-Muiño, M.A.; Osés, S.M.; Sancho, M.T.; Ibeas, S.; Reglero Ruiz, J.A.; Vallejos, S. Polymer film as starch azure container for the easy diastase activity determination in honey. Food Chem. 2021, 355, 129629. [Google Scholar] [CrossRef]
- Han, S.; Yang, J.; Choi, K.; Kim, J.; Adhikari, K.; Lee, J. Chemical Analysis of Commercial White Wines and Its Relationship with Consumer Acceptability. Foods 2022, 11, 603. [Google Scholar] [CrossRef]
- Fell, R.D. The color grading of honey. Am. Bee J. 1978, 18, 782–789. [Google Scholar]
- Aubert, S.; Gonnet, M. Mesure de la couleur des miels. Apidologie 1983, 14, 105–118. [Google Scholar] [CrossRef]
- Ortiz Valbuena, A.; Silva Losada, M.C. Caracterizacion cromatica (CIE L10, a10, b10) de las mieles de la alcarria y zonas adyacentes. Cuad. Apic. 1990, 8, 8–11. [Google Scholar]
- Persano Oddo, L.; Piazza, M.G.; Zellini, G. Caratteristiche 62. cromatiche dei mieli uniflorali. Apicoltura 1995, 63, 109–120. [Google Scholar]
- Hewavitharana, G.G.; Perera, D.N.; Navaratne, S.B.; Wickramasinghe, I. Extraction methods of fat from food samples and preparation of fatty acid methyl esters for gas chromatography: A review. Arab. J. Chem. 2020, 13, 6865–6875. [Google Scholar] [CrossRef]
- Chromý, V.; Vinklárková, B.; Šprongl, L.; Bittová, M. The Kjeldahl method as a primary reference procedure for total protein in certified reference materials used in clinical chemistry. I. A review of Kjeldahl methods adopted by laboratory medicine. Crit. Rev. Anal Chem. 2015, 45, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Evers, J.M.; Hughes, C.G. Chemical analysis. In Encyclopedia of Dairy Sciences; Roginski, H., Ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 34–39. [Google Scholar]
- Contini, M.; Baccelloni, S.; Massantini, R.; Anelli, G. Extraction of natural antioxidants from hazelnut (Corylus avellana L.) shell and skin wastes by long maceration at room temperature. Food Chem. 2008, 110, 659–669. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Development and validation of an HPTLC–DPPH assay and its application to the analysis of honey. JPCJ Planar Chromat. 2020, 33, 301–311. [Google Scholar] [CrossRef]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Advances in the analytical methods for determining the antioxidant properties of honey: A review. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Gorjanović, S.Ž.; Alvarez-Suarez, J.M.; Novaković, M.M.; Pastor, F.T.; Pezo, L.; Battino, M.; Sužnjević, D.Ž. Comparative analysis of antioxidant activity of honey of different floral sources using recently developed polarographic and various spectrophotometric assays. J. Food Compos. Anal. 2013, 30, 13–18. [Google Scholar] [CrossRef]
- Ou, B.; Chang, T.; Huang, D.; Prior, R.L. Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe: First action 2012.23. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oki, T.; Nagai, S.; Yoshinaga, M.; Nishiba, Y.; Suda, I. Contribution of β-carotene to radical scavenging capacity varies among orange-fleshed sweet potato cultivars. Food Sci. Technol. Res. 2006, 12, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Spanish Royal Decree-Law (RD) 1049/2003; BOE no. 186; Ministerio de la Presidencia: Madrid, Spain, 5 August 2003.
- Lafraxo, H.; Bakour, M.; Laaroussi, H.; El Ghouizi, A.; Ousaaid, D.; Aboulghazi, A.; Lyoussi, B. The Synergistic Beneficial Effect of Thyme Honey and Olive Oil against Diabetes and Its Complications Induced by Alloxan in Wistar Rats. Evid. Based Complement. Alternat. Med. 2021, 2021, 9949056. [Google Scholar] [CrossRef] [PubMed]
- Dag, B.; Sirali, R.; Tarakci, Z. Investigation of Some Properties of Chestnut Honey Produced in Black Sea Region Of Turkey. Batman Univ. J. Life Sci. 2017, 7, 118–123. [Google Scholar]
- Anjos, O.; Iglesias, C.; Peres, F.; Martínez, J.; García, Á.; Taboada, J. Neural networks applied to discriminate botanical origin of honeys. Food Chem. 2015, 175, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Abramovic, H.; Jamnik, M.; Burkan, L.; Kac, M. Water activity and water content in Slovenian honeys. Food Control 2008, 19, 1086–1090. [Google Scholar] [CrossRef]
- Fallico, B.; Zappala, M.; Arena, E.; Verzera, A. Effects of conditioning on HMF content in unifloral honeys. Food Chem. 2004, 85, 305–313. [Google Scholar] [CrossRef]
- Rodríguez, I.; Tananaki, C.; Galán-Soldevilla, H.; Pérez-Cacho, P.R.; Serrano, S. Sensory Profile of Greek Islands Thyme Honey. Appl. Sci. 2021, 11, 9548. [Google Scholar] [CrossRef]
- Akgün, N.; Çelik, Ö.F.; Kelebekli, L. Physicochemical properties, total phenolic content, and antioxidant activity of chestnut, rhododendron, acacia and multifloral honey. Food Meas. 2021, 15, 3501–3508. [Google Scholar] [CrossRef]
- Kolayli, S.; Can, Z.; Yildiz, O.; Sahin, H.; Karaoglu, S.A. A comparative study of the antihyaluronidase, antiurease, antioxidant, antimicrobial and physicochemical properties of different unifloral degrees of chestnut (Castanea sativa Mill.) honeys. J. Enzyme Inhib. Med. Chem. 2016, 31, 96–104. [Google Scholar] [CrossRef] [Green Version]
- León-Ruiz, V.; Vera, S.; González-Porto, A.V.; San Andrés, M.P. Vitamin C and sugar levels as simple markers for discriminating Spanish honey sources. J. Food Sci. 2011, 76, C356–C361. [Google Scholar] [CrossRef]
- Xagoraris, M.; Lazarou, E.; Kaparakou, E.H.; Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.S. Botanical origin discrimination of Greek honeys: Physicochemical parameters versus Raman spectroscopy. J. Sci. Food Agric. 2021, 101, 3319–3327. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tang, H. Honey on Basketball Players’ Physical Recovery and Nutritional Supplement. Comput. Intell. Neurosci. 2022, 2022, 6953568. [Google Scholar] [CrossRef]
- Mustafa, G.; Iqbal, A.; Javid, A.; Hussain, A.; Bukhari, S.M.; Ali, W.; Saleem, M.; Azam, S.M.; Sughra, F.; Ali, A.; et al. Variations in nutritional profile of honey produced by various species of genus Apis. Braz. J. Biol. 2021, 83, e246651. [Google Scholar] [CrossRef]
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Baigbabaei, A.; Shahidi, M. Determination of the floral origin of honey based on its phenolic profile and physicochemical properties coupled with chemometrics. Int. J. Food Prop. 2020, 23, 506–519. [Google Scholar] [CrossRef] [Green Version]
- Mok-Ryeon, A.; Kumazawa, S.; Usui, Y.; Nakamura, J.; Matsuka, M.; Zhu, F.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007, 101, 1383–1392. [Google Scholar] [CrossRef]
- León-Ruiz, V.; González-Porto, A.V.; Al-Habsi, N.; Vera, S.; San Andrés, M.P.; Jauregi, P. Antioxidant, antibacterial and ACE-inhibitory activity of four monofloral honeys in relation to their chemical composition. Food Funct. 2013, 4, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- D’Oliveira Sant’Ana, L.D.; Buarque Ferreira, A.B.; Lorenzon, M.C.A.; Berbara, R.L.L.; Castro, R.N. Correlation of total phenolic and flavonoid contents of Brazilian honeys with colour and antioxidant capacity. Int. J. Food Prop. 2014, 17, 65–76. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Argüelles, S. Bee products: Royal jelly and propolis. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Sanches Silva, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 475–484. [Google Scholar]
- Ozdal, T.; Ceylan, F.D.; Eroglu, N.; Kaplan, M.; Olgun, E.O.; Capanoglu, E. Investigation of antioxidant capacity, bioaccessibility and LC-MS/MS phenolic profile of Turkish propolis. Food Res. Int. 2019, 122, 528–536. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Yang, Y.C.; Shi, L.S.; Peng, C.C. Antioxidant properties of royal jelly associated with larval age and time of harvest. J. Agric. Food Chem. 2008, 56, 11447–11452. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, H.; Aydoğan, G.; Helvacioğlu, S.; Charehsaz, M.; Guzelmeric, E.; Aydin, A. Antioxidant, antiinflammatory and antimutagenic activities of various kinds of Turkish honey. FABAD J. Pharm. Sci. 2017, 42, 7. [Google Scholar]
- Sagdic, O.; Silici, S.; Ekici, L. Evaluation of the phenolic content, antiradical, antioxidant, and antimicrobial activity of different floral sources of honey. Int. J. Food Prop. 2013, 16, 658–666. [Google Scholar] [CrossRef]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Juszczak, L.; Gałkowska, D.; Ostrowska, M.; Socha, R. Antioxidant activity of honey supplemented with bee products. Nat. Prod. Res. 2015, 30, 1436–1439. [Google Scholar] [CrossRef]
- Gośliński, M.; Nowak, D.; Szwengiel, A. Multidimensional comparative analysis of bioactive phenolic compounds of honeys of various origin. Antioxidants 2021, 10, 530. [Google Scholar] [CrossRef]
- Valdés-Silverio, L.; Iturralde, G.; García-Tenesaca, M.; Paredes-Moreta, J.; Narváez-Narváez, D.; Rojas-Carrillo, M.; Tejera, E.; Beltrán-Ayala, P.; Giampieri, F.; Alvarez-Suarez, J. Physicochemical parameters, chemical composition, antioxidant capacity, microbial contamination and antimicrobial activity of Eucalyptus honey from the Andean region of Ecuador. J. Apic. Res. 2018, 57, 382–394. [Google Scholar] [CrossRef]
- Viktorova, J.; Stranska-Zachariasova, M.; Fenclova, M.; Vitek, L.; Hajslova, J.; Kren, V.; Ruml, T. Complex evaluation of antioxidant capacity of milk thistle dietary supplements. Antioxidants 2019, 8, 317. [Google Scholar] [CrossRef] [Green Version]
- Dawidowicz, A.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as antioxidant agents and their intervention abilities in antioxidant action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef]
- Kuskoski, E.; Asuero, A.; Troncoso, A.; Mancini-Filho, J.; Fett, R. Aplicação de diversos métodos químicos para determinar atividade antioxidante em polpa de frutas. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Rocchetti, G.; Castiglioni, S.; Maldarizzi, G.; Carloni, P.; Lucini, L. UHPLC-ESI-QTOF-MS phenolic profiling and antioxidant capacity of bee pollen from different botanical origin. Int. J. Food Sci. Technol. 2018, 54, 335–346. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, Y.J.; Baik, M.Y.; Kim, D.O.; Lee, H. Total phenolic contents and antioxidant activities of Korean domestic honey from different floral sources. Food Sci. Biotechnol. 2015, 24, 1453–1457. [Google Scholar] [CrossRef]
- Hailu, D.; Belay, A. Melissopalynology and antioxidant properties used to differentiate Schefflera abyssinica and polyfloral honey. PLoS ONE 2020, 15, e0240868. [Google Scholar] [CrossRef] [PubMed]
- Mokaya, H.O.; Bargul, J.L.; Irungu, J.W.; Lattorff, H.M.G. Bioactive constituents, in vitro radical scavenging and antibacterial activities of selected Apis mellifera honey from Kenya. Int. J. Food Sci. Technol. 2020, 55, 1246–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, L.; Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Evaluating the Presence and Contents of Phytochemicals in Honey Samples: Phenolic Compounds as Indicators to Identify Their Botanical Origin. Foods 2021, 10, 2616. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhong, S.; Yu, H.; Chen, P.; Li, B.; Li, D.; Liu, C.; Feng, Z. Covalent and Noncovalent Complexation of Phosvitin and Gallic Acid: Effects on Protein Functionality and In vitro Digestion Properties. J. Agric. Food Chem. 2022, 70, 11715–11726. [Google Scholar] [CrossRef]
- Djuardi, A.U.P.; Yuliana, N.D.; Ogawa, M.; Akazawa, T.; Suhartono, M.T. Emulsifying properties and antioxidant activity of soy protein isolate conjugated with tea polyphenol extracts. J. Food Sci. Technol. 2020, 57, 3591–3600. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Niu, F.; Li, J.; Su, Y.; Liu, Y.; Yang, Y. Interactions between tea polyphenol and two kinds of typical egg white proteins—ovalbumin and lysozyme: Effect on the gastrointestinal digestion of both proteins in vitro. Food Res. Int. 2014, 59, 100–107. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Topal, E.; Balkanska, R.; Yücel, B.; Oravecz, T.; Cornea-Cipcigan, M.; Vodnar, D.C. Monofloral Honeys as a Potential Source of Natural Antioxidants, Minerals and Medicine. Antioxidants 2021, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Song, I.B.; Han, H.J.; Lee, N.Y.; Cha, J.Y.; Son, Y.K.; Kwon, J. Antioxidant Activity of Royal Jelly Hydrolysates Obtained by Enzymatic Treatment. Korean J. Food Sci. Anim. Resour. 2018, 38, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Asgharpour, F.; Moghadamnia, A.A.; Motallebnejad, M.; Nouri, H.R. Propolis attenuates lipopolysaccharide-induced inflammatory responses through intracellular ROS and NO levels along with downregulation of IL-1β and IL-6 expressions in murine RAW 264.7 macrophages. J. Food Biochem. 2019, 43, e12926. [Google Scholar] [CrossRef]
- Kim, H.B.; Yoo, B.S. Propolis Inhibits UVA-Induced Apoptosis of Human Keratinocyte HaCaT Cells by Scavenging ROS. Toxicol. Res. 2016, 32, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braik, A.; Lahouel, M.; Merabet, R.; Djebar, M.R.; Morin, D. Myocardial protection by propolis during prolonged hypothermic preservation. Cryobiology 2019, 88, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Pfeiffer, P.; Alday, E.; Carreño, A.L.; Hernández-Tánori, J.; Montaño-Leyva, B.; Ortega-García, J.; Valdez, J.; Garibay-Escobar, A.; Hernandez, J.; Valencia, D.; et al. Seasonality Modulates the Cellular Antioxidant Activity and Antiproliferative Effect of Sonoran Desert Propolis. Antioxidants 2020, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Kucharska, A.Z.; Sokół-Łętowska, A.; Mertas, A.; Czuba, Z.P.; Król, W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evid. Based Complement. Alternat. Med. 2013, 2013, 976415. [Google Scholar] [CrossRef] [Green Version]
- Rippe, J.M.; Angelopoulos, T.J. Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding. Nutrients 2016, 8, 697. [Google Scholar] [CrossRef] [Green Version]
- Abadam, V.; Yeh, A.D. Sugar and Sweeteners Outlook: June 2022, SSS-M-406, U.S. Department of Agriculture, Economic Research Service. 2022. Available online: https://www.ers.usda.gov/publications/pub-details/?pubid=104127 (accessed on 22 August 2022).
- Castro-Muñoz, R.; Correa-Delgado, M.; Córdova-Almeida, R.; Lara-Nava, D.; Chávez-Muñoz, M.; Velásquez-Chávez, V.F.; Hernández-Torres, C.E.; Gontarek-Castro, E.; Ahmad, M.Z. Natural sweeteners: Sources, extraction and current uses in foods and food industries. Food Chem. 2022, 370, 130991. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, Y.; Naumovski, N.; Zhao, W.; Yang, G.; Xue, X.; Wu, L.; Granato, D.; Peng, W.; Wang, K. Systems Biology Approaches for Understanding Metabolic Differences Using ‘Multi-Omics’ Profiling of Metabolites in Mice Fed with Honey and Mixed Sugars. Nutrients 2022, 14, 3445. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, F.; Yang, C.; Zhao, C.; Cheng, N.; Cao, W.; Zhao, H. Alternative to Sugar, Honey Does Not Provoke Insulin Resistance in Rats Based on Lipid Profiles, Inflammation, and IRS/PI3K/AKT Signaling Pathways Modulation. J. Agric. Food Chem. 2022, 70, 10194–10208. [Google Scholar] [CrossRef]
- Sirisha, A.; Singh Gaur, G.; Pal, P.; Suryanarayana, B.S.; Bobby, Z.; Pal, G.K. Effects of Three Months of Honey Supplementation on Quality of Life and Neuropathy in Type 2 Diabetic Patients. Altern. Ther. Health Med. 2021, 27, 54–60. [Google Scholar] [PubMed]
- Roila, R.; Sordini, B.; Esposto, S.; Ranucci, D.; Primavilla, S.; Valiani, A.; Taticchi, A.; Branciari, R.; Servili, M. Effect of the Application of a Green Preservative Strategy on Minced Meat Products: Antimicrobial Efficacy of Olive Mill Wastewater Polyphenolic Extract in Improving Beef Burger Shelf-Life. Foods 2022, 11, 2447. [Google Scholar] [CrossRef] [PubMed]
- Bobade, H.; Singh, A.; Sharma, S.; Gupta, A.; Singh, B. Effect of extrusion processing on techno-functional, textural and bioactive properties of whole-grain corn flour-based breakfast cereals sweetened with honey. J. Texture Stud. 2022, 53, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Mafra, J.F.; de Santana, T.S.; Cruz, A.I.C.; Ferreira, M.A.; Miranda, F.M.; Araújo, F.M.; Ribeiro, P.R.; Evangelista-Barreto, N.S. Influence of red propolis on the physicochemical, microbiological and sensory characteristics of tilapia (Oreochromis niloticus) salami. Food Chem. 2022, 394, 133502. [Google Scholar] [CrossRef] [PubMed]
- Cedeño-Pinos, C.; Marcucci, M.C.; Bañón, S. Contribution of Green Propolis to the Antioxidant, Physical, and Sensory Properties of Fruity Jelly Candies Made with Sugars or Fructans. Foods 2021, 10, 2586. [Google Scholar] [CrossRef] [PubMed]




| Honey and Bee Products | Scientific and Common Name | Family | Geographic Region | Type |
|---|---|---|---|---|
| Thyme honey | Thymus spp. Thyme | Lamiaceae | Spain (Zamora) | Monofloral |
| Chestnut honey | Castanea sativa Chestnut | Fagaceae | Spain (Toledo) | Monofloral |
| Royal jelly | - | - | France | - |
| Propolis tincture * | - | - | Spain (Zamora) | - |
| Sample | Description | Code |
|---|---|---|
| 1 | Thyme honey | TH |
| 2 | Thyme honey + 2% royal jelly | TH + 2RJ |
| 3 | Thyme honey + 10% royal jelly | TH + 10RJ |
| 4 | Thyme honey + 2% propolis | TH + 2PR |
| 5 | Thyme honey + 10% propolis | TH + 10PR |
| 6 | Chestnut honey | CH |
| 7 | Chestnut honey + 2% royal jelly | CH + 2RJ |
| 8 | Chestnut honey + 10% royal jelly | CH + 10RJ |
| 9 | Chestnut honey + 2% propolis | CH + 2PR |
| 10 | Chestnut honey + 10% propolis | CH + 10PR |
| 11 | Thyme honey + 2% royal jelly + 2% propolis | TH + 2RJ + 2PR |
| 12 | Thyme honey + 10% royal jelly + 10% propolis | TH + 10RJ + 10PR |
| 13 | Chestnut honey + 2% royal jelly + 2% propolis | CH + 2RJ + 2PR |
| 14 | Chestnut honey + 10% royal jelly + 10% propolis | CH + 10RJ + 10PR |
| 15 | Royal jelly | RJ |
| 16 | Propolis | PR |
| 17 | Artificial honey | AH |
| Parameter | Thyme Honey | Chestnut Honey |
|---|---|---|
| pH (u) | 4.90 ± 0.01 | 5.31 ± 0.02 |
| Free acidity (meq/kg) | 28.50 ± 0.43 | 26.25 ± 0.35 |
| Moisture (%) | 16.65 ± 0.50 | 15.83 ± 0.44 |
| Electrical conductivity (mS/cm) | 0.64 ± 0.10 | 1.16 ± 0.05 |
| Water activity (u) | 0.53 ± 0.01 | 0.51 ± 0.01 |
| Hydroxymethylfurfural (mg/kg) | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Diastase (Schade units) | 21.10 ± 0.42 | 30.00 ± 0.55 |
| Glucose (g/100g) | 26.40 ± 0.12 | 25.80 ± 0.18 |
| Fructose (g/100g) | 37.40 ± 0.26 | 41.57 ± 0.15 |
| Glucose + Fructose (%) | 63.80 | 67.37 |
| Sucrose (g/100g) | 1.25 ± 0.08 | 0.68 ± 0.01 |
| X10 (u) | 0.50 ± 0.01 | 0.55 ± 0.02 |
| Y10 (u) | 0.46 ± 0.01 | 0.44 ± 0.01 |
| Z10 (u) | 0.04 ± 0.01 | 0.01 ± 0.01 |
| L*10 (u) | 66.90 ± 0.48 | 44.90 ± 0.39 |
| a*10 (u) | 16.20 ± 0.02 | 26.24 ± 0.12 |
| b*10 (u) | 81.79 ± 0.11 | 72.75 ± 0.09 |
| C*ab (u) | 83.38 ± 0.17 | 77.34 ± 0.20 |
| hab, 10 (u) | 78.79 ± 0.10 | 70.16 ± 0.18 |
| Pfund (u) | 83.00 ± 0.58 | 118.00 ± 1.10 |
| Turbidity (u) | 0.12 ± 0.01 | 0.26 ± 0.01 |
| Fat (%) | <0.50 | <0.50 |
| Protein (%) | 0.45 ± 0.01 | 0.61 ± 0.01 |
| Sample | Code | TPC (mg eq. GAE/100 g of Sample) | DPPH (µmol eq. Tx/g of Sample) | ORAC (µmol eq. Tx/g of Sample) | ABTS (µmol eq. Tx/g of Sample) |
|---|---|---|---|---|---|
| 1 | TH | 38.68 ± 5.93 cd | 1.37 ± 0.10 c–e | 4.17 ± 0.99 c | 7.36 ± 0.55 de |
| 2 | TH + 2RJ | 39.38 ± 3.75 d | 1.51 ± 0.16 de | 4.33 ± 0.53 c | 7.94 ± 0.56 ef |
| 3 | TH + 10RJ | 38.32 ± 1.67 cd | 1.30 ± 0.14 b–e | 4.34 ± 1.15 c | 6.64 ± 0.48 b–e |
| 4 | TH + 2PR | 52.27 ± 4.76 e | 2.61 ± 0.17 fg | 13.95 ± 0.63 e | 9.77 ± 0.72 fg |
| 5 | TH + 10PR | 107.42 ± 9.18 h | 7.19 ± 0.24 j | 19.04 ± 1.44 f | 25.96 ± 1.05 i |
| 6 | CH | 35.41 ± 2.14 cd | 1.09 ± 0.12 b–d | 3.59 ± 0.87 bc | 4.95 ± 0.30 bc |
| 7 | CH + 2RJ | 31.32 ± 1.71 cd | 0.96 ± 0.08 b–d | 3.38 ± 0.39 bc | 5.04 ± 0.41 bc |
| 8 | CH + 10RJ | 26.05 ± 1.77 bc | 0.72 ± 0.07 a–c | 5.05 ± 0.69 c | 4.46 ± 0.30 b |
| 9 | CH + 2PR | 38.87 ± 3.68 d | 1.43 ± 0.08 c–e | 10.47 ± 0.70 d | 6.87 ± 0.81 c–e |
| 10 | CH + 10PR | 72.83 ± 4.71 fg | 3.44 ± 0.17 h | 12.92 ± 0.78 de | 11.99 ± 0.31 gh |
| 11 | TH + 2RJ + 2PR | 39.75 ± 2.50 de | 1.98 ± 0.08 ef | 4.62 ± 0.41 c | 7.43 ± 0.50 de |
| 12 | TH + 10RJ + 10PR | 83.21 ± 3.95 g | 4.37 ± 0.19 i | 14.74 ± 1.30 e | 12.73 ± 0.69 h |
| 13 | CH + 2RJ + 2PR | 35.06 ± 1.56 cd | 1.14 ± 0.14 b–d | 5.27 ± 1.03 c | 5.36 ± 0.16 b–d |
| 14 | CH + 10RJ + 10PR | 66.23 ± 2.01 f | 2.88 ± 0.18 gh | 14.11 ± 1.05 e | 11.34 ± 0.27 gh |
| 15 | RJ | 17.21 ± 0.71 b | 0.63 ± 0.57 ab | 1.61 ± 0.78 ab | 1.89 ± 0.35 a |
| 16 | PR | 266.83 ± 19.80 i | 24.10 ± 1.17 k | 40.82 ± 0.56 g | 68.54 ± 3.91 j |
| 17 | AH | 0.81 ± 0.32 a | 0.16 ± 0.02 a | 0.03 ± 0.01 a | 0.08 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Martín, V.; Morales, P.; González-Porto, A.V.; Iriondo-DeHond, A.; López-Parra, M.B.; Del Castillo, M.D.; Hospital, X.F.; Fernández, M.; Hierro, E.; Haza, A.I. Enhancement of the Antioxidant Capacity of Thyme and Chestnut Honey by Addition of Bee Products. Foods 2022, 11, 3118. https://doi.org/10.3390/foods11193118
Sánchez-Martín V, Morales P, González-Porto AV, Iriondo-DeHond A, López-Parra MB, Del Castillo MD, Hospital XF, Fernández M, Hierro E, Haza AI. Enhancement of the Antioxidant Capacity of Thyme and Chestnut Honey by Addition of Bee Products. Foods. 2022; 11(19):3118. https://doi.org/10.3390/foods11193118
Chicago/Turabian StyleSánchez-Martín, Vanesa, Paloma Morales, Amelia V. González-Porto, Amaia Iriondo-DeHond, Marta B. López-Parra, María Dolores Del Castillo, Xavier F. Hospital, Manuela Fernández, Eva Hierro, and Ana I. Haza. 2022. "Enhancement of the Antioxidant Capacity of Thyme and Chestnut Honey by Addition of Bee Products" Foods 11, no. 19: 3118. https://doi.org/10.3390/foods11193118