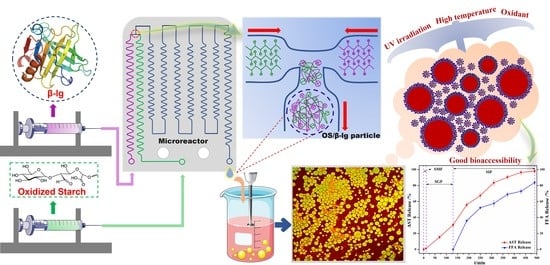
Preparation of Oxidized Starch/β-Lactoglobulin Complex Particles Using Microfluidic Chip for the Stabilization of Astaxanthin Emulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Stock Solution Preparation
2.3. Preparation of OS/β-lg Complex Coacervates through Microfluidic Platform
2.4. Preparation of OS/β-lg Particle-Stabilized Emulsions
2.5. Physical and Chemical Stability of AST-Enriched Emulsions
2.6. In Vitro Digestion of AST-Enriched Emulsions and Free Fatty Acid Release
2.7. Statistical Analysis
3. Results and Discussion
3.1. Structure Characterizations of Oxidized Starch and Commercial β-lg
3.2. OS/β-lg Complex Coacervation through Microfluidic Platform
3.3. Effects of pH, DS, and Mixing Ratio on OS/β-lg Complex Coacervation
3.4. Particulate Properties of OS/β-lg Complex Coacervates
3.5. Emulsifying Performance of OS/β-lg Complex Particles
3.5.1. Droplet Size and Microscopic Morphology
3.5.2. Microrheological Properties
3.5.3. Visualization of Interfacial Structure
3.5.4. Physical and Chemical Stability of AST-Enriched Emulsions
3.6. Gastrointestinal Fate of AST-Enriched Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, H.S.; Sung, D.K.; Kim, S.H.; Choi, W.I.; Hwang, E.T.; Choi, D.J.; Chang, J.H. Controlled release of astaxanthin from nanoporous silicified-phospholipids assembled boron nitride complex for cosmetic applications. Appl. Surf. Sci. 2017, 424, 15–19. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as vehicles for astaxanthin: Characterization, in vitro release evaluation and structure. Molecules 2018, 23, 2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; McClements, D.J.; Cao, Y.; Xiao, H. Chemical and physical stability of astaxanthin-enriched emulsion-based delivery systems. Food Biophys. 2016, 11, 302–310. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Wang, J.; Zou, L.; Deng, S.; Liu, W.; Yan, C.; Zhu, Y.; Cheng, C.; Liu, C. Coencapsulation of (−)-Epigallocatechin-3-gallate and quercetin in particle-stabilized W/O/W emulsion gels: Controlled release and bioaccessibility. J. Agric. Food. Chem. 2018, 66, 3691–3699. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure–function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
- Zhu, F. Starch based Pickering emulsions: Fabrication, properties, and applications. Trends Food Sci. Technol. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Piao, J.; Adachi, S. Stability of O/W emulsions prepared using various monoacyl sugar alcohols as an emulsifier. Innov. Food Sci. Emerg. Technol. 2006, 7, 211–216. [Google Scholar] [CrossRef]
- Hebishy, E.; Buffa, M.; Guamis, B.; Blasco-Moreno, A.; Trujillo, A.-J. Physical and oxidative stability of whey protein oil-in-water emulsions produced by conventional and ultra high-pressure homogenization: Effects of pressure and protein concentration on emulsion characteristics. Innov. Food Sci. Emerg. Technol. 2015, 32, 79–90. [Google Scholar] [CrossRef]
- Schmitt, C.; Bovay, C.; Vuilliomenet, A.M.; Rouvet, M.; Bovetto, L.; Barbar, R.; Sanchez, C. Multiscale characterization of individualized beta-lactoglobulin microgels formed upon heat treatment under narrow pH range conditions. Langmuir 2009, 25, 7899–7909. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Davis, J.P. Food protein functionality: A comprehensive approach. Food Hydrocoll. 2011, 25, 1853–1864. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, X.; Zhang, H.; Zhang, Y.; Ganzle, M.; Yang, N.; Nishinari, K.; Fang, Y. Probiotic encapsulation in water-in-water emulsion via heteroprotein complex coacervation of type-A gelatin/sodium caseinate. Food Hydrocoll. 2020, 105, 105790. [Google Scholar] [CrossRef]
- Gholamali, I.; Hosseini, S.N.; Alipour, E. Doxorubicin-loaded oxidized starch/poly (vinyl alcohol)/CuO bio-nanocomposite hydrogels as an anticancer drug carrier agent. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 967–980. [Google Scholar] [CrossRef]
- Tie, S.; Su, W.; Zhang, X.; Chen, Y.; Zhao, X.; Tan, M. pH-Responsive Core–Shell Microparticles Prepared by a Microfluidic Chip for the Encapsulation and Controlled Release of Procyanidins. J. Agric. Food. Chem. 2021, 69, 1466–1477. [Google Scholar] [CrossRef]
- Brzeziński, M.; Socka, M.; Kost, B. Microfluidics for producing polylactide nanoparticles and microparticles and their drug delivery application. Polym. Int. 2019, 68, 997–1014. [Google Scholar] [CrossRef]
- Valencia, P.M.; Basto, P.A.; Zhang, L.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Single-step assembly of homogenous lipid− polymeric and lipid− quantum dot nanoparticles enabled by microfluidic rapid mixing. ACS Nano 2010, 4, 1671–1679. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Rivera, M.M.; García-Suárez, F.J.L.; Del Valle, M.V.; Gutierrez-Meraz, F.; Bello-Pérez, L.A. Partial characterization of banana starches oxidized by different levels of sodium hypochlorite. Carbohydr. Polym. 2005, 62, 50–56. [Google Scholar] [CrossRef]
- Klassen, D.R.; Elmer, C.M.; Nickerson, M.T. Associative phase separation involving canola protein isolate with both sulphated and carboxylated polysaccharides. Food Chem. 2011, 126, 1094–1101. [Google Scholar] [CrossRef]
- Liu, W.; Gao, H.; McClements, D.J.; Zhou, L.; Wu, J.; Zou, L. Stability, rheology, and β-carotene bioaccessibility of high internal phase emulsion gels. Food Hydrocoll. 2019, 88, 210–217. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Soto-Añual, M.; Leal-Calderón, F.; Bustamante, M. Food-grade Pickering emulsion as a novel astaxanthin encapsulation system for making powder-based products: Evaluation of astaxanthin stability during processing, storage, and its bioaccessibility. Food Res. Int. 2020, 134, 109244. [Google Scholar] [CrossRef]
- Lee, G.; Chang, C.; Huang, S.; Yang, R. The hydrodynamic focusing effect inside rectangular microchannels. J. Micromech. Microeng. 2006, 16, 1024–1032. [Google Scholar] [CrossRef]
- Siow, L.-F.; Ong, C.-S. Effect of pH on garlic oil encapsulation by complex coacervation. J. Food Process. Technol 2013, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shim, Y.Y.; Shen, J.; Wang, Y.; Reaney, M.J. Whey protein isolate and flaxseed (Linum usitatissimum L.) gum electrostatic coacervates: Turbidity and rheology. Food Hydrocoll. 2017, 64, 18–27. [Google Scholar] [CrossRef]
- Lan, Y.; Ohm, J.-B.; Chen, B.; Rao, J. Phase behavior and complex coacervation of concentrated pea protein isolate-beet pectin solution. Food Chem. 2020, 307, 125536. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Wang, Y.; Reaney, M.J. Intermolecular interaction and complex coacervation between bovine serum albumin and gum from whole flaxseed (Linum usitatissimum L.). Food Hydrocoll. 2015, 49, 95–103. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Sun, N.; Wang, Y.; Lin, S. Formation and evaluation of casein-gum arabic coacervates via pH-dependent complexation using fast acidification. Int. J. Biol. Macromol. 2018, 120, 783–788. [Google Scholar] [CrossRef]
- Yang, Y.; Anvari, M.; Pan, C.-H.; Chung, D. Characterisation of interactions between fish gelatin and gum arabic in aqueous solutions. Food Chem. 2012, 135, 555–561. [Google Scholar] [CrossRef]
- Anal, A.K.; Tobiassen, A.; Flanagan, J.; Singh, H. Preparation and characterization of nanoparticles formed by chitosan–caseinate interactions. Colloids Surf. B. Biointerfaces 2008, 64, 104–110. [Google Scholar] [CrossRef]
- Su, J.; Guo, Q.; Chen, Y.; Dong, W.; Mao, L.; Gao, Y.; Yuan, F. Characterization and formation mechanism of lutein pickering emulsion gels stabilized by β-lactoglobulin-gum arabic composite colloidal nanoparticles. Food Hydrocoll. 2020, 98, 105276. [Google Scholar] [CrossRef]
- Taylor, P. Ostwald ripening in emulsions. Adv. Colloid Interface Sci. 1998, 75, 107–163. [Google Scholar] [CrossRef]
- Li, X.; Xie, Q.; Zhu, J.; Pan, Y.; Meng, R.; Zhang, B.; Chen, H.; Jin, Z. Chitosan hydrochloride/carboxymethyl starch complex nanogels as novel Pickering stabilizers: Physical stability and rheological properties. Food Hydrocoll. 2019, 93, 215–225. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.; Wei, Y.; Mao, L.; Gao, Y. Characterization of Pickering emulsion gels stabilized by zein/gum arabic complex colloidal nanoparticles. Food Hydrocoll. 2018, 74, 239–248. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C. Soy glycinin as food-grade Pickering stabilizers: Part. III. Fabrication of gel-like emulsions and their potential as sustained-release delivery systems for β-carotene. Food Hydrocoll. 2016, 56, 434–444. [Google Scholar] [CrossRef]
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 2: Stability and functionality. Food Hydrocoll. 2015, 49, 127–134. [Google Scholar] [CrossRef]
- Xiao, J.; Gonzalez, A.J.P.; Huang, Q. Kafirin nanoparticles-stabilized Pickering emulsions: Microstructure and rheological behavior. Food Hydrocoll. 2016, 54, 30–39. [Google Scholar] [CrossRef]
- Park, S.-A.; Ahn, J.-B.; Choi, S.-H.; Lee, J.-S.; Lee, H.G. The effects of particle size on the physicochemical properties of optimized astaxanthin-rich Xanthophyllomyces dendrorhous-loaded microparticles. LWT Food Sci. Technol. 2014, 55, 638–644. [Google Scholar] [CrossRef]
- Liu, C.; Tan, Y.; Xu, Y.; McCleiments, D.J.; Wang, D. Formation, characterization, and application of chitosan/pectin-stabilized multilayer emulsions as astaxanthin delivery systems. Int. J. Biol. Macromol. 2019, 140, 985–997. [Google Scholar] [CrossRef]
- Pu, J.; Bankston, J.D.; Sathivel, S. Developing microencapsulated flaxseed oil containing shrimp (Litopenaeus setiferus) astaxanthin using a pilot scale spray dryer. Biosys. Eng. 2011, 108, 121–132. [Google Scholar] [CrossRef]
- Xiao, J.; Li, C.; Huang, Q. Kafirin nanoparticle-stabilized Pickering emulsions as oral delivery vehicles: Physicochemical stability and in vitro digestion profile. J. Agric. Food. Chem. 2015, 63, 10263–10270. [Google Scholar] [CrossRef]
- Boonlao, N.; Shrestha, S.; Sadiq, M.B.; Anal, A.K. Influence of whey protein-xanthan gum stabilized emulsion on stability and in vitro digestibility of encapsulated astaxanthin. J. Food Eng. 2020, 272, 109859. [Google Scholar] [CrossRef]
- Luo, Q.; Borst, J.W.; Westphal, A.H.; Boom, R.M.; Janssen, A.E.M. Pepsin diffusivity in whey protein gels and its effect on gastric digestion. Food Hydrocoll. 2017, 66, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, W.; Salt, L.J.; Ridout, M.J.; Ding, Y.; Wilde, P.J. Fish oil emulsions stabilized with caseinate glycated by dextran: Physicochemical stability and gastrointestinal fate. J. Agric. Food. Chem. 2018, 67, 452–462. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Wu, Z.; Wang, Y.; Cheng, J.; Wang, T.; Zhang, B. Chitosan hydrochloride/carboxymethyl starch complex nanogels stabilized Pickering emulsions for oral delivery of β-carotene: Protection effect and in vitro digestion study. Food Chem. 2020, 315, 126288. [Google Scholar] [CrossRef]








| Particle Samples | Emulsions (MCT Oil Containing 0.1% AST, w/w) | |||||||
|---|---|---|---|---|---|---|---|---|
| Usage (%) | Flow Rate (mL/min) | Flow Ratio | pH | DS (%) | Mixing Ratio | Usage (%) | Oil/Water Ratio | Ionic Strength (mM) |
| 1.0 | 0.50 | 1:4 | 3.2 | 0.72 | 2:10 | 1 | 1:4 | 0 |
| 1.5 | 0.50 | 1:4 | 3.6 | 0.72 | 2:10 | 2 | 1:4 | 0 |
| 2.0 | 0.50 | 1:4 | 4.0 | 0.72 | 2:10 | 4 | 1:4 | 0 |
| 3.0 | 0.50 | 1:4 | 4.4 | 0.72 | 2:10 | 6 | 1:4 | 0 |
| 1.5 | 0.25 | 1:4 | 3.6 | 0.25 | 2:10 | 4 | 1:20 | 0 |
| 1.5 | 0.50 | 1:4 | 3.6 | 0.72 | 2:10 | 4 | 1:10 | 0 |
| 1.5 | 1.0 | 1:4 | 4 | 1:4 | 0 | |||
| 1.5 | 2.0 | 1:4 | 3.6 | 1.65 | 2:10 | 4 | 1:1 | 0 |
| 1.5 | 0.50 | 1:4 | 3.6 | 0.72 | 1:10 | 4 | 1:4 | 0 |
| 1.5 | 0.50 | 1:2 | 3.6 | 0.72 | 2:10 | 4 | 1:4 | 20 |
| 1.5 | 0.50 | 3:4 | 3.6 | 0.72 | 3:10 | 4 | 1:4 | 100 |
| 1.5 | 0.50 | 1:1 | 3.6 | 0.72 | 5:10 | 4 | 1:4 | 200 |
| pH | Images | CA (°) | Starch | Images | CA (°) | Mixing Ratio | Images | CA (°) |
|---|---|---|---|---|---|---|---|---|
| 4.4 |  | 73.52 | OS-0.25 |  | 109.38 | 1:10 |  | 112.63 |
| 4.0 |  | 81.68 | OS-0.72 |  | 86.68 | 2:10 |  | 86.68 |
| 3.6 |  | 86.68 | OS-1.65 |  | 61.14 | 3:10 |  | 81.26 |
| 3.2 |  | 77.49 | / | / | / | 5:10 |  | 72.21 |
| pH |
Interfacial Tension (mN/m) | OS | Interfacial Tension (mN/m) | Mixing Ratio |
Interfacial Tension (mN/m) |
|---|---|---|---|---|---|
| 4.4 | 7.18 b | OS-A | 10.16 b | 1:10 | 6.55 c |
| 4.0 | 6.66 c | OS-B | 4.84 c | 2:10 | 4.84 d |
| 3.6 | 4.84 d | OS-C | 18.97 a | 3:10 | 10.14 b |
| 3.2 | 14.11 a | / | / | 5:10 | 17.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Zhang, L.; Chen, L.; Li, X. Preparation of Oxidized Starch/β-Lactoglobulin Complex Particles Using Microfluidic Chip for the Stabilization of Astaxanthin Emulsion. Foods 2022, 11, 3078. https://doi.org/10.3390/foods11193078
Wang T, Zhang L, Chen L, Li X. Preparation of Oxidized Starch/β-Lactoglobulin Complex Particles Using Microfluidic Chip for the Stabilization of Astaxanthin Emulsion. Foods. 2022; 11(19):3078. https://doi.org/10.3390/foods11193078
Chicago/Turabian StyleWang, Tianxing, Lulu Zhang, Ling Chen, and Xiaoxi Li. 2022. "Preparation of Oxidized Starch/β-Lactoglobulin Complex Particles Using Microfluidic Chip for the Stabilization of Astaxanthin Emulsion" Foods 11, no. 19: 3078. https://doi.org/10.3390/foods11193078