Enhancing the Dispersion Stability and Sustained Release of S/O/W Emulsions by Encapsulation of CaCO3 Droplets in Sodium Caseinate/Xanthan Gum Microparticles
Abstract
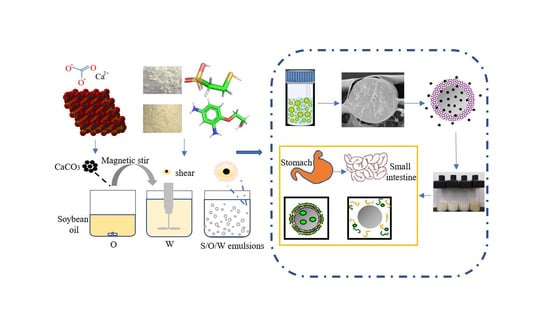
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of S/O/W Emulsions
2.3. Zeta-Potential, Particle Size, and Physical Stability Measurements
2.4. Viscosity Tests
2.5. Microstructure Analysis
2.5.1. Confocal Laser Scanning Microscopy (CLSM)
2.5.2. Cryo-Scanning Electron Microscopy
2.6. X-ray Diffraction (XRD)
2.7. Infrared Spectral
2.8. Raman Spectral Analysis
2.9. Molecular Interconnection
2.10. Simulated Gastrointestinal Tract (GIT) Model
2.10.1. Zeta-Potential and Particle Size of the Digested Samples
2.10.2. CLSM of the Digested Samples
2.10.3. SDS-PAGE
2.10.4. Determination of Free Calcium
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effects of XG Concentrations on CaCO3 S/O/W Emulsions
3.2. Effects of pH on CaCO3 S/O/W Emulsions
3.3. Viscosity Analysis
3.4. Microstructure Analysis
3.4.1. CLSM
3.4.2. Cryo-SEM
3.5. XRD Analysis
3.6. Infrared Spectral Analysis
3.7. Raman Spectrum Analysis
3.8. Molecular Docking Analysis
3.9. Digestion Characteristics Analysis
3.9.1. Particle Size and Zeta-Potential of Different Digestion Stages
3.9.2. Microstructure Analysis
3.9.3. SDS-PAGE Analysis
3.9.4. Determination of Free Calcium Release
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weaver, C.; Heaney, R.; Nickel, K.; Packard, P.I. Calcium bioavailability from high oxalate vegetables: Chinese vegetables, sweet potatoes and rhubarb. J. Food Sci. 1997, 62, 524–525. [Google Scholar] [CrossRef]
- Icen, H.; Guru, M. Extraction of caffeine from tea stalk and fiber wastes using supercritical carbon dioxide. J. Supercrit. Fluids 2009, 50, 225–228. [Google Scholar] [CrossRef]
- Kopic, S.; Geibel, J. Gastric acid, calcium absorption, and their impact on bone health. Physiol. Rev. 2013, 93, 189–268. [Google Scholar] [CrossRef]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef]
- Mcintyre, I.; O’Sullivan, M.; O’Riordan, D. Monitoring the progression of calcium and protein solubilization as affected by calcium chelators during small-scale manufacture of casein-based food matrices. Food Chem. 2017, 237, 597–604. [Google Scholar] [CrossRef]
- Paques, J.P.; Sagis, L.M.C.; van Rijn, C.J.M.; van der Linden, E. Nanospheres of alginate prepared through w/o emulsification and internal gelation with nanoparticles of CaCO3. Food Hydrocoll. 2014, 40, 182–188. [Google Scholar] [CrossRef]
- Guo, X.; Li, X.; Chan, L.; Huang, W.; Chen, T. Edible CaCO3 nanoparticles stabilized Pickering emulsion as calcium-fortified formulation. J. Nanobiotechnology 2021, 19, 67. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.-G.; Hao, J.; Li, X.-Y.; Xu, D.-X.; Cao, Y.-P. In vitro digestion of solid-in-oil-in-water emulsions for delivery of CaCO3. Food Hydrocoll. 2022, 129, 107605. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.-W.; Xu, D.-X.; Cao, Y.-P. Stability, Microstructure, and Rheological Properties of CaCO3 S/O/W Calcium-Lipid Emulsions. Foods 2021, 10, 2216. [Google Scholar] [CrossRef]
- Morita, T.; Sakamura, Y.; Horikiri, Y.; Suzuki, T.; Yoshino, H. Protein encapsulation into biodegradable microspheres by a novel S/O/W emulsion method using poly (ethylene glycol) as a protein micronization adjuvant. J. Control. Release 2000, 69, 435–444. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, J.; Zhong, Q.-X. S/O/W emulsions prepared with sugar beet pectin to enhance the viability of probiotic Lactobacillus salivarius NRRL B-30514. Food Hydrocoll. 2016, 52, 804–810. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Q. Freeze-dried capsules prepared from emulsions with encapsulated lactase as a potential delivery system to control lactose hydrolysis in milk. Food Chem. 2018, 214, 397–402. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, Q. Encapsulation of konjac glucomannan in oil droplets to reduce viscosity of aqueous suspensions and gradually increase viscosity during simulated gastric digestion. J. Food Eng. 2016, 175, 104–107. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, C.; Du, M.; Lin, S.; Xu, X.; Yu, P. In-situ dispersion of casein to form nanoparticles for Pickering high internal phase emulsions. LWT-Food Sci. Technol. 2020, 139, 110538. [Google Scholar] [CrossRef]
- Cao, Y.-H.; Dickinson, E.; Wedlock, D.J. Creaming and flocculation in emulsions containing polysaccharide. Food Hydrocoll. 1990, 4, 185–195. [Google Scholar] [CrossRef]
- Dickinson, E.; Ma, J.-G.; Povey, M.J.W. Creaming of concentrated oil-in-water emulsions containing xanthan. Food Hydrocoll. 1994, 8, 481–497. [Google Scholar] [CrossRef]
- Surh, J.; Decker, E.A.; McClements, D.J. Properties and stability of oil-in-water emulsions stabilized by fish gelatin. Food Hydrocoll. 2006, 20, 596–606. [Google Scholar] [CrossRef]
- Singh, H. Aspects of milk-protein-stabilised emulsions. Food Hydrocoll. 2011, 25, 1938–1944. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Zhao, Q.-Z.; Kong, J.; Zhao, M.-M. Effect of Xanthan gum on the stability of sodium caseinate emulsion. Sci. Technol. Food Ind. 2012, 33, 83–86. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, Q. Properties of whey protein-maltodextrin conjugates as impacted by powder acidity during the Maillard reaction. Food Hydrocoll. 2014, 38, 85–94. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, C.; Liu, Q.; Xu, C.; Xiong, Y.-L. Ca2+-selective electrode: A simple method to measure the phytase-aided release of bound calcium in soymilk. J. Food Compos. Anal. 2015, 39, 43–47. [Google Scholar] [CrossRef]
- McClements, D.J. Emulsion design to improve the delivery of functional lipophilic components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Cao, W.-C. Effect of Xanthan Gum and CMC on the Stability of Acidic Milk Drink. Master’s Thesis, Jiangnan University, Wuxi, China, 2006. [Google Scholar]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar] [CrossRef]
- Xiao, J.; Lu, X.; Huang, Q. Double emulsion derived from kafirin nanoparticles stabilized Pickering emulsion: Fabrication, microstructure, stability and in vitro digestion profile. Food Hydrocoll. 2017, 62, 230–238. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Zhao, Q.-Z.; Liu, T.-X.; Long, Z.; Kong, J.; Zhao, M.-M. Sodium caseinate/xanthan gum interactions in aqueous solution: Effect on protein adsorption at the oil-water interface. Food Hydrocoll. 2012, 27, 339–346. [Google Scholar]
- Zhang, G.-S.; Yuan, C.; Yang, C.-Y.; Han, B. Study on the prevention of osteoporosis by collagen polypeptide calcium. Sci. Technol. Food Ind. 2010, 31, 346–348. [Google Scholar]
- Vagenas, N.V.; Gatsouli, A.; Kontoyannis, C.G. Quantitative analysis of synthetic calcium carbonate polymorphs using FT-IR spectroscopy. Talanta 2003, 59, 831–836. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, M.; Ma, M. Emulsifying properties of ovalbumin: Improvement and mechanism by phosphorylation in the presence of sodium tripolyphosphate. Food Hydrocoll. 2016, 60, 29–37. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G. Spectroscopic characterization of natural calcite minerals. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2007, 68, 656–664. [Google Scholar] [CrossRef]
- Ren, H.-W.; Zhang, W.-S.; Li, X.-Y.; Liu, N. Effect of pH on circular dichroism and Raman spectroscopy of secondary structure of β-casein from Chinese human milk. Spectrosc. Spectr. Anal. 2015, 35, 384–389. [Google Scholar]
- Wang, J. Study on the Stability and Digestive Properties of β -Carotene Emulsion Hydrogel Particles. Master’s Thesis, Nanchang University, Nanchang, China, 2018; pp. 39–45. [Google Scholar]
- He, F.-Y.; Lin, H. Structure, properties and application of xanthan gum in food processing. J. Guangxi Vocat. Tech. Coll. 2009, 2, 1–4. [Google Scholar]
- Waraho, T.; McClements, D.-J.; Decker, E.-A. Impact of free fatty acid concentration and structure on lipid oxidation in oil-in-water emulsions. Food Chem. 2011, 129, 854–859. [Google Scholar] [CrossRef]
- Lueamsaisuk, C.; Lentle, R.-G.; MacGibbon, A.-K.-H.; Matia-Merino, L.; Golding, M. The effect of lactoferrin on physical changes in phospholipid stabilised emulsions during neonatal in vitro gastric digestion: Does synergism of pepsin and lipase promote lipolysis in protein-stabilised emulsions. Food Hydrocoll. 2015, 43, 785–793. [Google Scholar]
- Wu, H.-J.; Sun, J.-X.; Yu, Z.-Y. Preparation and application of amino acid and mineral chelates. J. Hengshui Univ. 2021, 23, 18–23. [Google Scholar]
- Wang, J.; Munk, M.-B.; Skibsted, L.-H.; Ahrne, L.-M. Impact of pectin and whey minerals solubilized by lime juice on calcium bioaccessibility in yogurt based snacks. Food Hydrocoll. 2022, 131, 107817. [Google Scholar] [CrossRef]
- Cong, L.; Lu, G.-H. Calcium preparation correct selection and reasonable application. Chin. Mod. Drug Appl. 2010, 4, 128. [Google Scholar]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, G.; Xu, D.; Cao, Y. Enhancing the Dispersion Stability and Sustained Release of S/O/W Emulsions by Encapsulation of CaCO3 Droplets in Sodium Caseinate/Xanthan Gum Microparticles. Foods 2022, 11, 2854. https://doi.org/10.3390/foods11182854
Zhang J, Li G, Xu D, Cao Y. Enhancing the Dispersion Stability and Sustained Release of S/O/W Emulsions by Encapsulation of CaCO3 Droplets in Sodium Caseinate/Xanthan Gum Microparticles. Foods. 2022; 11(18):2854. https://doi.org/10.3390/foods11182854
Chicago/Turabian StyleZhang, Jie, Gongwei Li, Duoxia Xu, and Yanping Cao. 2022. "Enhancing the Dispersion Stability and Sustained Release of S/O/W Emulsions by Encapsulation of CaCO3 Droplets in Sodium Caseinate/Xanthan Gum Microparticles" Foods 11, no. 18: 2854. https://doi.org/10.3390/foods11182854