Sterilizing Ready-to-Eat Poached Spicy Pork Slices Using a New Device: Combined Radio Frequency Energy and Superheated Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. PSPS Preparation
2.2. Bacterial Culture and Spore Crop Preparation
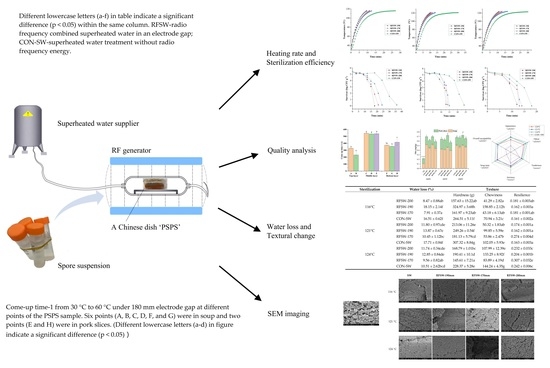
2.3. RFSW Heating System
2.4. The Cold Spot and Heating Rate
2.5. Inactivation of G. stearothermophilus Spores
2.5.1. Spore Inoculation into PSPS
2.5.2. The Sterilization of RFSW
2.5.3. Enumeration of Surviving Cells
2.6. Quality Analysis
2.6.1. Water Loss
2.6.2. Texture Measurement
2.6.3. Measurement of Thiobarbituric Acid Reactants (TBARS)
2.6.4. Sensory Evaluation
2.7. Microstructure Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determination of Cold Spot
3.2. The Heating Rate and Curve
3.3. Inactivation of G. stearothermophilus spores
3.4. Quality Evaluation
3.4.1. Water Loss
3.4.2. Texture Measurement
3.4.3. Oxidation Analysis
3.4.4. Sensory Evaluation
3.4.5. Microstructure Analysis on SEM Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osman, I.; Osman, S.; Mokhtar, I.; Setapa, F.; Shukor, S.A.M.; Temyati, Z. Family Food Consumption: Desire towards Convenient Food Products. Procedia-Soc. Behav. Sci. 2014, 121, 223–231. [Google Scholar] [CrossRef]
- Tirloni, E.; Nauta, M.; Vasconi, M.; Di Pietro, V.; Bernardi, C.; Stella, S. Growth of Listeria monocytogenes in ready-to-eat “shrimp cocktail”: Risk assessment and possible preventive interventions. Int. J. Food Microbiol. 2020, 334, 108800. [Google Scholar] [CrossRef] [PubMed]
- Carballo, D.; Moltó, J.C.; Berrada, H.; Ferrer, E. Presence of mycotoxins in ready-to-eat food and subsequent risk assessment. Food Chem. Toxicol. 2018, 121, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, L.; Carvalho, C.T.; Tavares, R.O.; de Mello Medeiros, V.; de Oliveira Rosas, C.; Silva, J.N.; dos Reis Lopes, S.M.; Forsythe, S.J.; Brandão, M.L.L. Isolation, molecular and phenotypic characterization of Cronobacter spp. in ready-to-eat salads and foods from Japanese cuisine commercialized in Brazil. Food Res. Int. 2018, 107, 353–359. [Google Scholar] [CrossRef]
- Horita, C.N.; Baptista, R.C.; Caturla, M.Y.R.; Lorenzo, J.M.; Barba, F.J.; Sant’Ana, A.S. Combining reformulation, active packaging and non-thermal post-packaging decontamination technologies to increase the microbiological quality and safety of cooked ready-to-eat meat products. Trends Food Sci. Technol. 2018, 72, 45–61. [Google Scholar] [CrossRef]
- Bedane, T.F.; Pedrós-Garrido, S.; Quinn, G.; Lyng, J.G. The impact of emerging domestic and commercial electro-heating technologies on energy consumption and quality parameters of cooked beef. Meat Sci. 2021, 179, 108550. [Google Scholar] [CrossRef]
- Yildiz-Turp, G.; Sengun, I.Y.; Kendirci, P.; Icier, F. Effect of ohmic treatment on quality characteristic of meat: A review. Meat Sci. 2013, 93, 441–448. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Herrero, A.M.; Cofrades, S.; Ruiz-Capillas, C. Meat: Eating Quality and Preservation. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 685–692. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wei, X.; Wu, D.; Dai, J.; Liu, S.; Qin, W. Effect of radio frequency-assisted hot-air drying on drying kinetics and quality of Sichuan pepper (Zanthoxylum bungeanum Maxim.). LWT 2021, 147, 111572. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, B.; Zhou, X.; Wang, S. Effects of combined radio frequency with hot water blanching on enzyme inactivation, color and texture of sweet potato. Innov. Food Sci. Emerg. Technol. 2020, 66, 102513. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, Y.; Cui, B.; Fu, H.; Chen, X.; Wang, Y. Radio frequency energy inactivates peroxidase in stem lettuce at different heating rates and associate changes in physiochemical properties and cell morphology. Food Chem. 2021, 342, 128360. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, C.; Sun, Y.; Zhang, X.; Wang, Y.; Fu, H.; Chen, X.; Wang, Y. Blanching effects of radio frequency heating on enzyme inactivation, physiochemical properties of green peas (Pisum sativum L.) and the underlying mechanism in relation to cellular microstructure. Food Chem. 2021, 345, 128756. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Kou, X.; Liu, L.; Hou, L.; Li, R.; Wang, S. Effect of water, fat, and salt contents on heating uniformity and color of ground beef subjected to radio frequency thawing process. Innov. Food Sci. Emerg. Technol. 2021, 68, 102604. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, S.; Zhang, M.; Cao, P.; Adhikari, B. Combined radio frequency and hot water pasteurization of Nostoc sphaeroides: Effect on temperature uniformity, nutrients content, and phycocyanin stability. LWT 2021, 141, 110880. [Google Scholar] [CrossRef]
- Dag, D.; Singh, R.K.; Kong, F. Effect of surrounding medium on radio frequency (RF) heating uniformity of corn flour. J. Food Eng. 2021, 307, 110645. [Google Scholar] [CrossRef]
- Wang, C.; Kou, X.; Zhou, X.; Li, R.; Wang, S. Effects of layer arrangement on heating uniformity and product quality after hot air assisted radio frequency drying of carrot. Innov. Food Sci. Emerg. Technol. 2021, 69, 102667. [Google Scholar] [CrossRef]
- Yang, Y.; Geveke, D.J. Shell egg pasteurization using radio frequency in combination with hot air or hot water. Food Microbiol. 2020, 85, 103281. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.Y.; Balasubramaniam, V.M. Inactivation of Geobacillus stearothermophilus spores in low-acid foods by pressure-assisted thermal processing. J. Sci. Food Agric. 2015, 95, 174–178. [Google Scholar] [CrossRef]
- Wang, K.; Huang, L.; Xu, Y.; Cui, B.; Sun, Y.; Ran, C.; Fu, H.; Chen, X.; Wang, Y.; Wang, Y. Evaluation of Pilot-Scale Radio Frequency Heating Uniformity for Beef Sausage Pasteurization Process. Foods 2022, 11, 1317. [Google Scholar] [CrossRef]
- Cui, B.; Sun, Y.; Wang, K.; Liu, Y.; Fu, H.; Wang, Y.; Wang, Y. Pasteurization mechanism on the cellular level of radio frequency heating and its possible non-thermal effect. Innov. Food Sci. Emerg. Technol. 2022, 78, 103026. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, K.; Dong, Y.; Li, K.; Liu, H.; Cui, B.; Fu, H.; Chen, X.; Wang, Y.; Wang, Y. Effects of radiofrequency blanching on lipoxygenase inactivation, physicochemical properties of sweet corn (Zea mays L.), and its correlation with cell morphology. Food Chem. 2022, 394, 133498. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, Z.; Dai, R. Effects of carnosine on color stability and lipid oxidation of beef patties. Sci. Technol. Food Ind. 2009, 30, 140–143. [Google Scholar] [CrossRef]
- Gillespie, R.; Ahlborn, G.J. Mechanical, sensory, and consumer evaluation of ketogenic, gluten-free breads. Food Sci. Nutr. 2021, 9, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Marra, F.; Wang, S. A novel strategy for improving radio frequency heating uniformity of dry food products using computational modeling. Innov. Food Sci. Emerg. Technol. 2016, 34, 100–111. [Google Scholar] [CrossRef]
- Rubalya Valantina, S. Measurement of dielectric constant: A recent trend in quality analysis of vegetable oil—A review. Trends Food Sci. Technol. 2021, 113, 1–11. [Google Scholar] [CrossRef]
- Zell, M.; Lyng, J.G.; Cronin, D.A.; Morgan, D.J. Ohmic cooking of whole beef muscle—Evaluation of the impact of a novel rapid ohmic cooking method on product quality. Meat Sci. 2010, 86, 258–263. [Google Scholar] [CrossRef]
- Gao, M.; Tang, J.; Wang, Y.; Powers, J.; Wang, S. Almond quality as influenced by radio frequency heat treatments for disinfestation. Postharvest Biol. Technol. 2010, 58, 225–231. [Google Scholar] [CrossRef]
- Tiwari, G.; Wang, S.; Tang, J.; Birla, S.L. Analysis of radio frequency (RF) power distribution in dry food materials. J. Food Eng. 2011, 104, 548–556. [Google Scholar] [CrossRef]
- Finley, N.; Fields, M.L. Heat activation and heat-induced dormancy of Bacillus stearothermophilus spores. Appl. Microbiol. 1962, 10, 231–236. [Google Scholar] [CrossRef]
- Georget, E.; Kushman, A.; Callanan, M.; Ananta, E.; Heinz, V.; Mathys, A. Geobacillus stearothermophilus ATCC 7953 spore chemical germination mechanisms in model systems. Food Control 2015, 50, 141–149. [Google Scholar] [CrossRef]
- N’Gatta, K.C.; Kondjoyan, A.; Favier, R.; Sicard, J.; Rouel, J.; Gruffat, D.; Mirade, P.-S. Impact of Combining Tumbling and Sous-Vide Cooking Processes on the Tenderness, Cooking Losses and Colour of Bovine Meat. Processes 2022, 10, 1229. [Google Scholar] [CrossRef]
- Palka, K.; Daun, H. Changes in texture, cooking losses, and myofibrillar structure of bovine M. semitendinosus during heating. Meat Sci. 1999, 51, 237–243. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Li, H.; Zhou, J.; Han, J.; Wei, C. Changes in the volatile profile, fatty acid composition and oxidative stability of flaxseed oil during heating at different temperatures. LWT 2021, 151, 112137. [Google Scholar] [CrossRef]
- Addis, P.B. Occurrence of lipid oxidation products in foods. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1986, 24, 1021–1030. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Medina-Meza, I.; Candoğan, K.; Bermúdez-Aguirre, D. Advanced retorting, microwave assisted thermal sterilization (MATS), and pressure assisted thermal sterilization (PATS) to process meat products. Meat Sci. 2014, 98, 420–434. [Google Scholar] [CrossRef]
- Traore, S.; Aubry, L.; Gatellier, P.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Santé-Lhoutellier, V. Effect of heat treatment on protein oxidation in pig meat. Meat Sci. 2012, 91, 14–21. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Du, M.; Shen, Q.W.; Zhang, D. Dephosphorylation enhances postmortem degradation of myofibrillar proteins. Food Chem. 2018, 245, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, D.; Xu, W.; Gao, F.; Zhou, G. Effect of final cooked temperature on tenderness, protein solubility and microstructure of duck breast muscle. LWT-Food Sci. Technol. 2013, 51, 266–274. [Google Scholar] [CrossRef]









| Soup | Pork Slices | ||||
|---|---|---|---|---|---|
| Dosage (g) | Information | Dosage (g) | Information | ||
| Spring onion | 15.0 ± 0.1 | Hao&Duo supermarket (Used stem only) | Salt | 1.50 ± 0.05 | Hao&Duo supermarket |
| Ginger | 5.0 ± 0.1 | Hao&Duo supermarket (Removed the peel) | Chinese rice wine | 2.0 ± 0.1 | Jingzhiliaojiu, Beijing Ershang Wangzhihe Food Co., Ltd., Beijing, China |
| Garlic | 7.0 ± 0.2 | Hao&Duo supermarket (Removed the peel) | Egg white | 9.0 ± 0.5 | Hao&Duo supermarket |
| Bean paste | 15.0 ± 0.1 | Pixian Bean paste, Pixian Star Seasoning Co., Ltd., Pixian, China | Potato starch | 9.0 ± 0.5 | Xi’an Panfeng Shunda Trading Co., Ltd., Xi’an, China |
| Salt | 4.0 ± 0.1 | Hao&Duo supermarket | |||
| Chicken extract | 0.40 ± 0.05 | Hao&Duo supermarket | |||
| Sugar | 4.0 ± 0.1 | Hao&Duo supermarket | |||
| Light soy sauce | 2.0 ± 0.1 | Haday soy sauce, Foshan Haitian Condiment Food Co., Ltd., Foshan, China | |||
| Chinese rice wine | 2.0 ± 0.1 | Jingzhiliaojiu, Beijing Ershang Wangzhihe Food Co., Ltd., Beijing, China | |||
| Water | 400 ± 1 | Laboratory drinking water (Food grade) | |||
| Superheated Water Temperature (°C) | Electrode Gaps (mm) | Come-Up Time-2 (min) | Heating Rate (°C/min) |
|---|---|---|---|
| 116 | 160 | 12.09 ± 0.52 | 7.12 |
| 170 | 11.35 ± 0.03 | 7.58 | |
| 180 | 10.26 ± 0.05 | 8.38 | |
| 190 | 9.57 ± 0.87 | 8.99 | |
| 200 | 12.70 ± 0.81 | 6.77 | |
| 121 | 160 | 13.25 ± 0.07 | 6.87 |
| 170 | 11.54 ± 0.48 | 7.89 | |
| 180 | 10.63 ± 0.88 | 8.56 | |
| 190 | 10.19 ± 0.51 | 8.93 | |
| 200 | 13.61 ± 0.56 | 6.69 | |
| 124 | 160 | 13.54 ± 0.13 | 6.94 |
| 170 | 13.05 ± 0.41 | 7.23 | |
| 180 | 11.62 ± 0.14 | 8.10 | |
| 190 | 11.12 ± 0.14 | 8.47 | |
| 200 | 16.43 ± 0.11 | 5.72 |
| Sterilization | Water Loss (%) | Texture | |||
|---|---|---|---|---|---|
| Hardness (g) | Chewiness | Resilience | |||
| 116 °C | RFSW-200 | 8.47 ± 0.88 ab | 157.63 ± 15.22 ab | 41.29 ± 2.82 a | 0.181 ± 0.003 ab |
| RFSW-190 | 18.15 ± 2.14 f | 324.97 ± 3.68 h | 158.85 ± 2.12 h | 0.162 ± 0.003 a | |
| RFSW-170 | 7.91 ± 0.37 a | 161.97 ± 9.23 ab | 43.18 ± 6.13 ab | 0.181 ± 0.001 ab | |
| CON-SW | 16.51 ± 0.62 f | 264.51 ± 5.11 f | 70.94 ± 3.21 c | 0.161 ± 0.002 a | |
| 121 °C | RFSW-200 | 11.80 ± 0.97 cde | 213.06 ± 11.26 e | 50.32 ± 1.83 ab | 0.174 ± 0.001 a |
| RFSW-190 | 13.87 ± 0.67 e | 249.26 ± 0.54 f | 99.85 ± 5.59 e | 0.162 ± 0.001 a | |
| RFSW-170 | 10.45 ± 1.12 bc | 181.13 ± 5.79 cd | 53.86 ± 2.47 b | 0.274 ± 0.004 d | |
| CON-SW | 17.71 ± 0.84 f | 307.32 ± 8.84 g | 102.05 ± 5.93 e | 0.163 ± 0.003 a | |
| 124 °C | RFSW-200 | 11.74 ± 0.34 cde | 168.79 ± 1.01 bc | 107.99 ± 12.39 e | 0.232 ± 0.033 c |
| RFSW-190 | 12.85 ± 0.84 de | 190.41 ± 10.1 d | 133.25 ± 8.92 f | 0.204 ± 0.001 b | |
| RFSW-170 | 9.56 ± 0.82 ab | 145.61 ± 7.21 a | 83.89 ± 4.19 d | 0.307 ± 0.032 e | |
| CON-SW | 10.51 ± 2.62 bcd | 228.37 ± 5.28 e | 144.24 ± 4.35 g | 0.242 ± 0.006 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Ran, C.; Cui, B.; Sun, Y.; Fu, H.; Chen, X.; Wang, Y.; Wang, Y. Sterilizing Ready-to-Eat Poached Spicy Pork Slices Using a New Device: Combined Radio Frequency Energy and Superheated Water. Foods 2022, 11, 2841. https://doi.org/10.3390/foods11182841
Wang K, Ran C, Cui B, Sun Y, Fu H, Chen X, Wang Y, Wang Y. Sterilizing Ready-to-Eat Poached Spicy Pork Slices Using a New Device: Combined Radio Frequency Energy and Superheated Water. Foods. 2022; 11(18):2841. https://doi.org/10.3390/foods11182841
Chicago/Turabian StyleWang, Ke, Chuanyang Ran, Baozhong Cui, Yanan Sun, Hongfei Fu, Xiangwei Chen, Yequn Wang, and Yunyang Wang. 2022. "Sterilizing Ready-to-Eat Poached Spicy Pork Slices Using a New Device: Combined Radio Frequency Energy and Superheated Water" Foods 11, no. 18: 2841. https://doi.org/10.3390/foods11182841