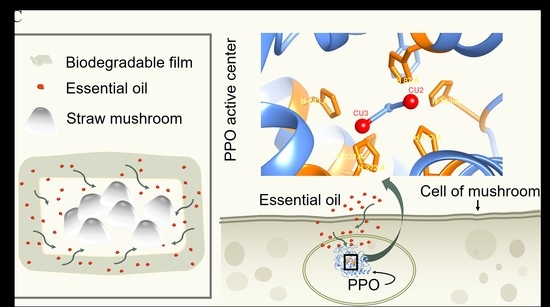
Active Antioxidant Packaging from Essential Oils Incorporated Polylactic Acid/Poly(butylene adipate-co-terephthalate)/Thermoplastic Starch for Preserving Straw Mushroom
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Characterization of Film
2.3.1. Mechanical Properties
2.3.2. Morphological Characterization
2.3.3. Water Vapor Transmission Rate (WVTR) and Oxygen Transmission Rate (OTR)
2.3.4. Antioxidant Capacity
2.4. Treatment of Straw Mushrooms
2.5. Qualities of Straw Mushrooms
2.5.1. PPO and Total Phenol Content (TPC)
2.5.2. Moisture Status
2.5.3. Autolysis Rate
2.5.4. Rate of Weight Loss (RWL)
2.5.5. Hardness
2.5.6. Total Soluble Solids (TSS)
2.5.7. Microbiological Analysis
2.6. Statistical Analyses
3. Results and Discussion
3.1. Film Characterization
3.1.1. Mechanical Properties
3.1.2. Morphological Characterization
3.1.3. WVTR and OTR
3.1.4. Antioxidant Capacity
3.2. Quality of Straw Mushrooms
3.2.1. PPO and TPC
3.2.2. Moisture Status (LF-NMR and MRI)
3.2.3. Autolysis Rate and RWL
3.2.4. Hardness and TSS
3.2.5. Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, D.L.; Yu, K.L.; Deng, Z.L.; Hu, Q.H.; Zhao, L.Y. Storage quality and flavor evaluation of Volvariella volvacea packaged with nanocomposite-based packaging material during commercial storage condition. Food Packag. Shelf Life 2019, 22, 9. [Google Scholar]
- Dhalsamant, K.; Dash, S.K.; Bal, L.M.; Panda, M.K. Effect of perforation mediated MAP on shelf life of mushroom (Volvariella volvacea). Sci. Hortic. 2015, 189, 41–50. [Google Scholar] [CrossRef]
- Kshanaprava, D.; Sanjaya, K.D.; Lalit, M.B.; Nihar, R.S. Effect of natural antimicrobials (clove and garlic) on shelf life and quality of mushroom (Volvariella volvacea) under modified atmosphere. J. Packag. Technol. Res. 2018, 2, 243–249. [Google Scholar]
- Li, N.; Chen, F.M.; Cui, F.J.; Sun, W.J.; Zhang, J.S.; Qian, L.S.; Yang, Y.; Wu, D.; Dong, Y.; Jiang, J.X.; et al. Improved postharvest quality and respiratory activity of straw mushroom (Volvariella volvacea) with ultrasound treatment and controlled relative humidity. Sci. Hortic. 2017, 225, 56–64. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Ferulic acid incorporated active films based on poly(lactide)/poly(butylene adipate-co-terephthalate) blend for food packaging. Food Packag. Shelf Life 2020, 24, 7. [Google Scholar] [CrossRef]
- Owolabi, I.O.; Songsamoe, S.; Matan, N. Combined impact of peppermint oil and lime oil on Mangosteen (Garcinia Mangostana) fruit ripening and mold growth using closed system. Postharvest Biol. Technol. 2021, 175, 9. [Google Scholar] [CrossRef]
- Qu, T.T.; Li, B.B.; Huang, X.F.; Li, X.X.; Ding, Y.; Chen, J.F.; Tang, X.M. Effect of Peppermint Oil on the Storage Quality of White Button Mushrooms (Agaricus bisporus). Food Bioprocess Technol. 2020, 13, 404–418. [Google Scholar] [CrossRef]
- Yang, C.; An, X.; Wan, J.; Zheng, T.; Bao, B.; Wei, Y.; Zhu, X.; Ouyang, Z. Antibacterial and antioxidant activities of different chemotypes of essential oils from Mentha haplocalyx Briq. Food Sci. Technol. 2021, 46, 185–192. [Google Scholar]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Oz, M.; El Nebrisi, E.G.; Yang, K.H.S.; Howarth, F.C.; Al Kury, L.T. Cellular and Molecular Targets of Menthol Actions. Front. Pharmacol. 2017, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.N.; Ren, L.P.; Li, M.L.; Qian, J.; Fan, J.F.; Du, B. Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef]
- Kong, X.; Liu, X.; Li, J.-y.; Yang, Y.-j. Advance in pharmacological research of eugenol. Hubei Agric. Sci. 2013, 52, 508–511. [Google Scholar]
- Jiang, T.J.; Luo, Z.S.; Ying, T.J. Fumigation with essential oils improves sensory quality and enhanced antioxidant ability of shiitake mushroom (Lentinus edodes). Food Chem. 2015, 172, 692–698. [Google Scholar] [CrossRef]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R. Effects of Essential Oil Vapour Treatment on the Postharvest Disease Control and Different Defence Responses in Two Mango (Mangifera indica L.) Cultivars. Food Bioprocess Technol. 2017, 10, 1131–1141. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Z.; Cai, H.; Wang, L.; Hu, C.; Li, D.; Chen, Y.; Kang, Y.; Li, L. Controlled moisture permeability of thermoplastic starch/polylactic acid/poly butylene adipate-co-terephthalate film for the autolysis of straw mushroom Volvariella volvacea. Food Chem. 2022, 373, 131409. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Paosen, S.; Vongkamjan, K.; Voravuthikunchai, S.P. Enhancement of food shelf life with polyvinyl alcohol-chitosan nanocomposite films from bioactive Eucalyptus leaf extracts. Food Biosci. 2020, 36, 13. [Google Scholar] [CrossRef]
- Go, E.J.; Bin Song, K. Capsosiphon fulvescens films containing persimmon (Diospyros kaki L.) leaf extract. Food Biosci. 2020, 37, 7. [Google Scholar] [CrossRef]
- Atares, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- ASTM-D882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM-E398-13; Standard Test Method for Water Vapor Transmission Rate of Sheet Materials Using Dynamic Relative Humidity Measurement. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM-D1434-82; Standard Test Method for Determining Gas Permeability Characteristics of Plastic Film and Sheeting. ASTM International: West Conshohocken, PA, USA, 2015.
- Louis, E.; Villalobos-Carvajal, R.; Reyes-Parra, J.; Jara-Quijada, E.; Ruiz, C.; Andrades, P.; Gacitua, J.; Beldarrain-Iznaga, T. Preservation of mushrooms (Agaricus bisporus) by an alginate-based-coating containing a cinnamaldehyde essential oil nanoemulsion. Food Packag. Shelf Life 2021, 28, 10. [Google Scholar] [CrossRef]
- Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Guidances of Physiological and Biochemical Experiments of Post-Harvest Fruits and Vegetables; China Light Industry Press: Beijing, China, 2007. [Google Scholar]
- Xiao, L.; Zhang, S.; Zhou, X.; Zhou, X.; Wu, H.; Li, S.; Shen, G.; Zhang, Z. Preparation and properties of corn starch-based antimicrobial films incorporated with cinnamon essential oil. Food Sci. China 2019, 40, 40–45. [Google Scholar]
- Azevedo, V.M.; Carvalho, R.A.; Borges, S.V.; Claro, P.I.C.; Hasegawa, F.K.; Yoshida, M.I.; Marconcini, J.M. Thermoplastic starch/whey protein isolate/rosemary essential oil nanocomposites obtained by extrusion process: Antioxidant polymers. J. Appl. Polym. Sci. 2019, 136, 12. [Google Scholar] [CrossRef]
- Brandelero, R.P.H.; de Almeida, F.M.; Alfaro, A. The microstructure and properties of starch-polyvinyl alcohol-alginate films with copaiba and lemongrass oils. Quim. Nova 2015, 38, 910–916. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Marzieh, P.; Hassan, B.; Mohammad, H. Production and evaluation of properties of edible starch film containing bene (Pistacia atlantica) gum essential oil. J. Res. Innov. Food Sci. Technol. 2017, 6, 25–38. [Google Scholar]
- Jia, X. Preparation and Properties of Maize Starch-Based Film Materials. Master’s Thesis, Jiangnan University, Wuxi, China, 2018. [Google Scholar]
- Aguilar-Sanchez, R.; Munguia-Perez, R.; Reyes-Jurado, F.; Navarro-Cruz, A.R.; Cid-Perez, T.S.; Hernandez-Carranza, P.; Beristain-Bauza, S.D.; Ochoa-Velasco, C.E.; Avila-Sosa, R. Structural, Physical, and Antifungal Characterization of Starch Edible Films Added with Nanocomposites and Mexican Oregano (Lippia berlandieri Schauer) Essential Oil. Molecules 2019, 24, 2340. [Google Scholar] [CrossRef] [Green Version]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef]
- Gulcin, I.; Elmastas, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Alparslan, Y. Antimicrobial and antioxidant capacity of biodegradable gelatin film forming solutions incorporated with different essential oils. J. Food Meas. Charact. 2018, 12, 317–322. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, J.H.; Zhu, M.H.; Gan, Z.L.; Ni, Y.-Y. Advances in research of the structure and browning mechanism of polyphenol oxidase. Food Res. Dev. 2015, 36, 113–119. [Google Scholar]
- Liu, J.; Liu, S.; Zhang, X.; Kan, J.; Jin, C.H. Effect of gallic acid grafted chitosan film packaging on the postharvest quality of white button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 147, 39–47. [Google Scholar] [CrossRef]
- Kurniawan, C.W.; Atmaka, W.; Manuhara, G.J.; Sanjaya, A.P. Quality Characteristic of Liquid Smoked Straw Mushroom (Volvariella volvacea) Ball during Storage. In Proceedings of the 2nd International Symposium on Food and Agro-Biodiversity (ISFA), Semarang, Indonesia, 26–27 September 2017; IOP Publishing Ltd.: Semarang, Indonesia, 2017. [Google Scholar]
- Li, M.; Li, B.; Zhang, W.J. Rapid and non-invasive detection and imaging of the hydrocolloid-injected prawns with low-field NMR and MRI. Food Chem. 2018, 242, 16–21. [Google Scholar] [CrossRef]
- Wenlong, S.; Yangyang, L.; Haiyan, G.; Yongfei, H.; Li, L. Effect of ginger essential oil microcapsule film packaging on okra preservation. Food Ferment. Ind. 2020, 46, 142–148. [Google Scholar]
- Li, L.; Song, W.L.; Shen, C.H.; Dong, Q.F.; Wang, Y.F.; Zuo, S. Active packaging film containing oregano essential oil microcapsules and their application for strawberry preservation. J. Food Process Preserv. 2020, 44, 13. [Google Scholar] [CrossRef]
- Gong, J.L. Study on Freshness Preservation Technology and Autolysis Mechanism of Pleurotus eryngii. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2013. [Google Scholar]
- Liu, Q.; Kong, W.L.; Hu, S.J.; Kang, Y.C.; Zhang, Y.T.; Ng, T.B. Effects of Oudemansiella radicata polysaccharide on postharvest quality of oyster mushroom (Pleurotus ostreatus) and its antifungal activity against Penicillium digitatum. Postharvest Biol. Technol. 2020, 166, 10. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Zhu, Y.Q.; Li, K.; Yang, D.; Xu, R.; Li, H.J. Study on postharvest and ripening quality of “Jinyan” kiwifruit with different maturation. Storage Process 2020, 20, 34–40. [Google Scholar]
- Zhu, Z.; Chen, Y.L.; Shi, G.Q.; Zhang, X.J. Selenium delays tomato fruit ripening by inhibiting ethylene biosynthesis and enhancing the antioxidant defense system. Food Chem. 2017, 219, 179–184. [Google Scholar] [CrossRef] [PubMed]







| Sample | TS (MPa) | EB (%) |
|---|---|---|
| PLA/PBAT/TPS | 8.54 ± 0.29 a | 235 ± 9.80 b |
| PLA/PBAT/TPS-PO | 6.53 ± 0.47 b | 150 ± 12.0 a |
| PLA/PBAT/TPS-CO | 5.40 ± 0.83 c | 167 ± 17.9 a |
| Sample | WVTR (g m−2 24 h−1) | OTR (cm3 m−2 day−1 0.1 MPa−1) |
|---|---|---|
| PLA/PBAT/TPS | 916 ± 58.1 a | 976 ± 2.73 a |
| PLA/PBAT/TPS-PO | 1036 ± 59.9 b | 1037 ± 64.9 ab |
| PLA/PBAT/TPS-CO | 1082 ± 61.8 b | 1130 ± 63.7 b |
| Sample | DPPH (%) |
|---|---|
| PLA/PBAT/TPS | 14.9 ± 0.8 a |
| PLA/PBAT/TPS-PO | 56.0 ± 7.8 b |
| PLA/PBAT/TPS-CO | 91.3 ± 1.5 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, H.; Zhao, M.; Zhang, S.; Yin, R.; Hu, C.; Fan, M.; Li, L. Active Antioxidant Packaging from Essential Oils Incorporated Polylactic Acid/Poly(butylene adipate-co-terephthalate)/Thermoplastic Starch for Preserving Straw Mushroom. Foods 2022, 11, 2252. https://doi.org/10.3390/foods11152252
Gui H, Zhao M, Zhang S, Yin R, Hu C, Fan M, Li L. Active Antioxidant Packaging from Essential Oils Incorporated Polylactic Acid/Poly(butylene adipate-co-terephthalate)/Thermoplastic Starch for Preserving Straw Mushroom. Foods. 2022; 11(15):2252. https://doi.org/10.3390/foods11152252
Chicago/Turabian StyleGui, Hang, Meiyan Zhao, Shiqi Zhang, Ruoyu Yin, Changying Hu, Min Fan, and Li Li. 2022. "Active Antioxidant Packaging from Essential Oils Incorporated Polylactic Acid/Poly(butylene adipate-co-terephthalate)/Thermoplastic Starch for Preserving Straw Mushroom" Foods 11, no. 15: 2252. https://doi.org/10.3390/foods11152252