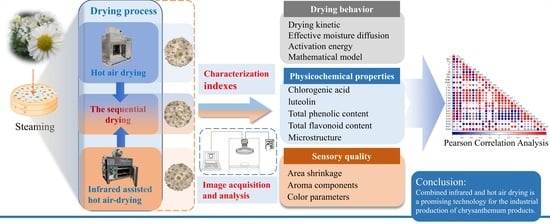
Effect of Combined Infrared and Hot Air Drying Strategies on the Quality of Chrysanthemum (Chrysanthemum morifolium Ramat.) Cakes: Drying Behavior, Aroma Profiles and Phenolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drying of Chrysanthemum Cakes
2.2.1. Freeze-Drying (FD)
2.2.2. Hot Air Drying (HAD)
2.2.3. Combined Infrared and Hot Air Drying (IR-HAD)
2.2.4. The Sequential Drying (IR-HAD + HAD)
2.3. Drying Characteristics
2.3.1. Moisture Ratio (MR) and Drying Rate (DR)
2.3.2. Effective Moisture Diffusivity (Deff)
2.3.3. Activation Energy (Ea)
2.4. Mathematical Modeling
2.5. Specific Energy Consumption
2.6. Appearance of Chrysanthemum Cakes
2.6.1. Shrinkage Ratio
2.6.2. Color Properties
2.7. GC-MS Analysis
2.7.1. Principal Component Analysis (PCA) of Volatile Compounds
2.7.2. Hierarchical Cluster Analysis (HCA) of Volatile Compounds
2.8. Determination of Phenolic Compounds
2.8.1. Extraction of Phenolic Compounds
2.8.2. UPLC-MS/MS Analysis of Phenolic Compounds
2.8.3. Total Phenolic Content (TPC)
2.8.4. Total Flavonoid Content (TFC)
2.9. Water Binding Capacity (WBC) and Water Holding Capacity (WHC)
2.10. Microstructure Analysis
2.11. Statistical Analysis
3. Results
3.1. Drying Kinetics
3.1.1. Moisture Ratio (MR) and Drying Rate (DR)
3.1.2. Mathematical Modeling
3.1.3. Moisture Diffusivity (Deff) and Activation Energy (Ea)
3.2. Specific Energy Consumption (SEC)
3.3. Effect of Drying Strategies on Shrinkage Ratio (SR)
3.4. Effect of Drying Strategies on Color Properties
3.5. Effect of Drying Strategies on Volatile Compounds
3.6. HCA and PCA of Volatile Compounds
3.7. Effect of Drying Strategies on Physicochemical Properties
3.7.1. Water Binding Capacity (WBC) and Water Holding Capacity (WHC)
3.7.2. Effect of Drying Strategies on Phenolic Compounds
3.8. Effect of Drying Strategies on Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, X.F.; Chu, J.Z.; Zhang, Y.F.; Liu, C.Q.; Yao, X.Q. Nutritional and active ingredients of medicinal chrysanthemum flower heads affected by different drying methods. Ind. Crops Prod. 2017, 104, 45–51. [Google Scholar] [CrossRef]
- Wang, T.; Xi, M.; Guo, Q.; Wang, L.; Shen, Z. Chemical components and antioxidant activity of volatile oil of a Compositae tea (Coreopsis tinctoria Nutt.) from Mt. Kunlun. Ind. Crops Prod. 2015, 67, 318–323. [Google Scholar] [CrossRef]
- Zhang, N.; He, Z.; He, S.; Jing, P. Insights into the importance of dietary chrysanthemum flower (Chrysanthemum morifolium cv. Hangju)-wolfberry (Lycium barbarum fruit) combination in antioxidant and anti-inflammatory properties. Food Res. Int. 2019, 116, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Meng, Q.; Luo, H.; Chen, F.; Zhou, Y.; He, M. Transcriptional responses for biosynthesis of flavor volatiles in methyl jasmonate-treated Chrysanthemum indicum var. aromaticum leaves. Ind. Crops Prod. 2020, 147, 112254. [Google Scholar] [CrossRef]
- Abraham-Juarez, M.D.; Olalde-Portugal, V.; Ceron-Garcia, A.; Sosa-Morales, M.E. Hot Air Drying Kinetics of Thin Layers of Prickly Pear Fruit Paste. Sains Malays. 2019, 48, 361–367. [Google Scholar] [CrossRef]
- Zhou, X.; Ramaswamy, H.; Qu, Y.; Xu, R.; Wang, S. Combined radio frequency-vacuum and hot air drying of kiwifruits: Effect on drying uniformity, energy efficiency and product quality. Innov. Food Sci. Emerg. Technol. 2019, 56, 102182. [Google Scholar] [CrossRef]
- Jin, J.; Yurkow, E.J.; Adler, D.; Lee, T.C. Improved freeze drying efficiency by ice nucleation proteins with ice morphology modification. Food Res. Int. 2018, 106, 90–97. [Google Scholar] [CrossRef]
- Kim, W.W.; Ghimeray, A.K.; Wu, J.C.; Eom, S.H.; Lee, B.G.; Kang, W.S.; Cho, D.H. Effect of Far Infrared Drying on Antioxidant Property, Anti-inflammatory Activity, and Inhibitory Activity in A549 Cells of Gamguk (Chrysanthemum indicum L.) Flower. Food Sci. Biotechnol. 2012, 21, 261–265. [Google Scholar] [CrossRef]
- Xiao, H.W.; Pan, Z.; Deng, L.Z.; El-Mashad, H.M.; Yang, X.H.; Mujumdar, A.S.; Gao, Z.J.; Zhang, Q. Recent developments and trends in thermal blanchin—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Li, B.; Lin, J.; Zheng, Z.; Duan, H.; Li, D.; Wu, M. Effects of different drying methods on drying kinetics and physicochemical properties of Chrysanthemum morifolium Ramat. Int. J. Agric. Biol. Eng. 2019, 12, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Bassey, E.J.; Cheng, J.H.; Sun, D.W. Novel nonthermal and thermal pretreatments for enhancing drying performance and improving quality of fruits and vegetables. Trends Food Sci. Technol. 2021, 112, 137–148. [Google Scholar] [CrossRef]
- Guclu, G.; Keser, D.; Kelebek, H.; Keskin, M.; Sekerli, Y.E.; Soysal, Y.; Selli, S. Impact of production and drying methods on the volatile and phenolic characteristics of fresh and powdered sweet red peppers. Food Chem. 2021, 338, 128129. [Google Scholar] [CrossRef]
- Hnin, K.K.; Zhang, M.; Ju, R.; Wang, B. A novel infrared pulse-spouted freeze drying on the drying kinetics, energy consumption and quality of edible rose flowers. LWT Food Sci. Technol. 2021, 136, 110318. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Qin, L.; Shen, J.; He, Z.; Shao, Q.; Lin, D. Drying methods affect bioactive compound contents and antioxidant capacity of Bletilla striata (Thunb.) Reichb.f. flower. Ind. Crops Prod. 2021, 164, 113388. [Google Scholar] [CrossRef]
- Alwi, H.; Zubairy, N.; Ku Hamid, K.H. Drying properties of agarwood leaves in vacuum far infrared dryer: Colour changes. Mater. Today Proc. 2022, 63, S174–S177. [Google Scholar] [CrossRef]
- Peng, J.; Yin, X.; Jiao, S.; Wei, K.; Tu, K.; Pan, L. Air jet impingement and hot air-assisted radio frequency hybrid drying of apple slices. LWT Food Sci. Technol. 2019, 116, 108517. [Google Scholar] [CrossRef]
- Qin, J.; Wang, Z.; Wang, X.; Shi, W. Effects of microwave time on quality of grass carp fillets processed through microwave combined with hot-air drying. Food Sci. Nutr. 2020, 8, 4159–4171. [Google Scholar] [CrossRef]
- Xiong, X.; Cao, X.; Zeng, Q.; Yang, X.; Wang, Y.; Zhang, R.; Huang, F.; Dong, L.; Zhang, M.; Su, D. Effects of heat pump drying and superfine grinding on the composition of bound phenolics, morphology and microstructure of lychee juice by-products. LWT Food Sci. Technol. 2021, 144, 111206. [Google Scholar] [CrossRef]
- Su, X.; Hurley, K.; Xu, Z.; Xu, Y.; Rutto, L.; O’Keefe, S.; Scoggins, H.; Yin, Y. Performance of alternative drying techniques on hop (Humulus lupulus L.) aroma quality: An HS-SPME-GC-MS-O and chemometrics combined approach. Food Chem. 2020, 381, 132289. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.; Castello-Cogollos, L.; Aleixandre, J.; Aleixandre-Benavent, R. Chemometrics in food science and technology: A bibliometric study. Chemom. Intell. Lab. Syst. 2022, 222, 104514. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Farag, N.F.; El-Ahmady, S.H.; Abdelrahman, E.H.; Naumann, A.; Schulz, H.; Azzam, S.M.; El-Kashoury, E.-S.A. Characterization of essential oils from Myrtaceae species using ATR-IR vibrational spectroscopy coupled to chemometrics. Ind. Crops Prod. 2018, 124, 870–877. [Google Scholar] [CrossRef]
- Oliveira, C.C.D.; Calado, V.M.D.A.; Ares, G.; Granato, D. Statistical approaches to assess the association between phenolic compounds and the in vitro antioxidant activity of Camellia sinensis and Ilex paraguariensis teas. Crit. Rev. Food Sci. Nutr. 2015, 55, 1456–1473. [Google Scholar] [CrossRef]
- Ghoshal, S.; Mattea, C.; Denner, P.; Stapf, S. Effect of Initial Conformation on the Starch Biopolymer Film Formation Studied by NMR. Molecules 2020, 25, 1227. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Duan, H.; Wang, Z.; Su, Y.; Zheng, Z.; Duan, Q. Design and experiment of infrared-hot air combined dryer based on temperature and humidity control. Trans. CSAM 2020, 51, 483–492. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, G.; Li, X.; Zhao, Y.; Lei, D.; Ding, G.; Ambrose, K.; Liu, Y. Combined medium- and short-wave infrared and hot air impingement drying of sponge gourd (Luffa cylindrical) slices. J. Food Eng. 2020, 284, 110043. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Effect of ultrasound pre-treatment on the drying kinetics of brown seaweed Ascophyllum nodosum. Ultrason. Sonochem. 2015, 23, 302–307. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Y.H.; Gao, Z.J.; Xie, Y.K.; Wang, H. Computer vision online measurement of shiitake mushroom (Lentinus edodes) surface wrinkling and shrinkage during hot air drying with humidity control. J. Food Eng. 2021, 292, 110253. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Abdan, K.; Janius, R.; Chen, G.N. The effectiveness of combined infrared and hot-air drying strategies for sweet potato. J. Food Eng. 2019, 241, 75–87. [Google Scholar] [CrossRef]
- Sun, D.; Wu, M.; Xu, H.; Shang, N.; Gao, F.; Wang, Y.; Zheng, Z. The bioactive properties and quality attributes of Chrysanthemum morifolium Ramat. as affected by pulsed vacuum drying. Dry. Technol. 2021, 39, 1–15. [Google Scholar] [CrossRef]
- Cebi, N.; Arici, M.; Sagdic, O. The famous Turkish rose essential oil: Characterization and authenticity monitoring by FTIR, Raman and GC-MS techniques combined with chemometrics. Food Chem. 2021, 354, 129495. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Beta, T. Discrimination of geographical origin of Napirira bean (Phaseolus vulgaris L.) based on phenolic profiles and antioxidant activity. J. Food Compos. Anal. 2017, 62, 217–222. [Google Scholar] [CrossRef]
- Escobar, S.; Santander, M.; Zuluaga, M.; Chacon, I.; Rodriguez, J.; Vaillant, F. Fine cocoa beans production: Tracking aroma precursors through a comprehensive analysis of flavor attributes formation. Food Chem. 2021, 365, 130627. [Google Scholar] [CrossRef] [PubMed]
- Major, N.; Goreta Ban, S.; Urlic, B.; Ban, D.; Dumicic, G.; Perkovic, J. Morphological and Biochemical Diversity of Shallot Landraces Preserved along the Croatian Coast. Front. Recent Dev. Plant. Sci. 2018, 9, 1749. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A. Variation in bioactive compounds, antioxidant and antibacterial activity of Iranian Chrysanthemum morifolium cultivars and determination of major polyphenolic compounds based on HPLC analysis. J. Food Sci. Technol. 2021, 58, 1538–1548. [Google Scholar] [CrossRef]
- Dabrosca, B.; Pacifico, S.; Cefarelli, G.; Mastellone, C.; Fiorentino, A. ‘Limoncella’ apple, an Italian apple cultivar: Phenolic and flavonoid contents and antioxidant activity. Food Chem. 2007, 104, 1333–1337. [Google Scholar] [CrossRef]
- Su, D.B.; Lv, W.Q.; Wang, Y.; Wang, L.J.; Li, D. Influence of microwave hot-air flow rolling dry-blanching on microstructure, water migration and quality of Pleurotus eryngii during hot-air drying. Food Control. 2020, 114, 107228. [Google Scholar] [CrossRef]
- Doymaz, İ.; Kocayigit, F. Drying and Rehydration Behaviors of Convection Drying of Green Peas. Dry. Technol. 2011, 29, 1273–1282. [Google Scholar] [CrossRef]
- Huang, X.; Li, W.; Wang, Y.; Wan, F. Drying characteristics and quality of Stevia rebaudiana leaves by far-infrared radiation. LWT Food Sci. Technol. 2021, 140, 110638. [Google Scholar] [CrossRef]
- Dehghannya, J.; Farshad, P.; Heshmati, M.K. Three-stage hybrid osmotic–intermittent microwave–convective drying of apple at low temperature and short time. Dry. Technol. 2018, 36, 1982–2005. [Google Scholar] [CrossRef]
- Balzarini, M.F.; Reinheimer, M.A.; Ciappini, M.C.; Scenna, N.J. Comparative study of hot air and vacuum drying on the drying kinetics and physicochemical properties of chicory roots. J. Food Sci. Technol. 2018, 55, 4067–4078. [Google Scholar] [CrossRef]
- Sahoo, M.; Titikshya, S.; Aradwad, P.; Kumar, V.; Naik, S.N. Study of the drying behaviour and color kinetics of convective drying of yam (Dioscorea hispida) slices. Ind. Crops Prod. 2022, 176, 114258. [Google Scholar] [CrossRef]
- Zarein, M.; Samadi, S.H.; Ghobadian, B. Investigation of microwave dryer effect on energy efficiency during drying of apple slices. J. Saudi Soc. Agric. Sci. 2015, 14, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, M.; Michalska, A. Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruits: Drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem. 2016, 212, 671–680. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Chen, X.; Li, B.; Mao, X.; Miao, J.; Zhao, C.; Huang, L.; Gao, W. Effects of hot air and microwave-assisted drying on drying kinetics, physicochemical properties, and energy consumption of chrysanthemum. Chem. Eng. Process. 2018, 129, 84–94. [Google Scholar] [CrossRef]
- Xiao, H.W.; Bai, J.W.; Xie, L.; Sun, D.W.; Gao, Z.J. Thin-layer air impingement drying enhances drying rate of American ginseng (Panax quinquefolium L.) slices with quality attributes considered. Food Bioprod. Process. 2015, 94, 581–591. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Mujumdar, A.S.; Chen, H. Drying and quality characteristics of shredded squid in an infrared-assisted convective dryer. Dry. Technol. 2014, 32, 1828–1839. [Google Scholar] [CrossRef]
- Mahiuddin, M.; Khan, M.I.H.; Kumar, C.; Rahman, M.M.; Karim, M.A. Shrinkage of Food Materials during Drying: Current Status and Challenges. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1113–1126. [Google Scholar] [CrossRef] [Green Version]
- Onwude, D.I.; Hashim, N.; Abdan, K.; Janius, R.; Chen, G. The potential of computer vision, optical backscattering parameters and artificial neural network modelling in monitoring the shrinkage of sweet potato (Ipomoea batatas L.) during drying. J. Sci. Food Agric. 2018, 98, 1310–1324. [Google Scholar] [CrossRef]
- Aral, S.; Bese, A.V. Convective drying of hawthorn fruit (Crataegus spp.): Effect of experimental parameters on drying kinetics, color, shrinkage, and rehydration capacity. Food Chem. 2016, 210, 577–584. [Google Scholar] [CrossRef]
- Xiao, H.W.; Law, C.L.; Sun, D.W.; Gao, Z.J. Color Change Kinetics of American Ginseng (Panax quinquefolium) Slices During Air Impingement Drying. Dry. Technol. 2014, 32, 418–427. [Google Scholar] [CrossRef]
- Wang, H.; Fang, X.M.; Sutar, P.P.; Meng, J.S.; Wang, J.; Yu, X.L.; Xiao, H.W. Effects of vacuum-steam pulsed blanching on drying kinetics, colour, phytochemical contents, antioxidant capacity of carrot and the mechanism of carrot quality changes revealed by texture, microstructure and ultrastructure. Food Chem. 2021, 338, 127799. [Google Scholar] [CrossRef]
- Gong, J.; Weng, Q.; Sun, J.; Wang, D.; Qiu, S.; Li, L.; Chu, B.; Xiao, G.; Liu, S.; Zheng, F. Steam Explosion Pretreatment Alters the Composition of Phenolic Compounds and Antioxidant Capacities in Chrysanthemum morifolium Ramat cv. ‘Hangbaiju’. J. Food Process. Preserv. 2021, 45, e15376. [Google Scholar] [CrossRef]
- Senadeera, W.; Adiletta, G.; Onal, B.; Di Matteo, M.; Russo, P. Influence of Different Hot Air Drying Temperatures on Drying Kinetics, Shrinkage, and Colour of Persimmon Slices. Foods 2020, 9, 101. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wongso, I.; Putnam, D.; Khir, R.; Pan, Z. Effect of hot air and infrared drying on the retention of cannabidiol and terpenes in industrial hemp (Cannabis sativa L.). Ind. Crops Prod. 2021, 172, 114051. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Luo, D.; Wu, J.; Ma, Z.; Tang, P.; Liao, X.; Lao, F. Production of high sensory quality Shiitake mushroom (Lentinus edodes) by pulsed air-impingement jet drying (AID) technique. Food Chem. 2021, 341, 128290. [Google Scholar] [CrossRef]
- Kebede, B.; Grauwet, T.; Andargie, T.; Sempiri, G.; Palmers, S.; Hendrickx, M.; Van Loey, A. Kinetics of Strecker aldehyde formation during thermal and high pressure high temperature processing of carrot puree. Innov. Food Sci. Emerg. Technol. 2017, 39, 88–93. [Google Scholar] [CrossRef]
- Mounir, S. Texturing of Chicken Breast Meat as an Innovative Way to Intensify Drying: Use of a Coupled Washing/Diffusion CWD Phenomenological Model to Enhance Kinetics and Functional Properties. Dry. Technol. 2015, 33, 1369–1381. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, Z.; Bi, J.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Degradation kinetics of total phenolic compounds, capsaicinoids and antioxidant activity in red pepper during hot air and infrared drying process. Int. J. Food Sci. Technol. 2016, 51, 842–853. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Huarte, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.P. Influence of heat treatment on antioxidant capacity and (poly) phenolic compounds of selected vegetables. Food Chem. 2016, 197, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Kulapichitr, F.; Borompichaichartkul, C.; Fang, M.; Suppavorasatit, I.; Cadwallader, K.R. Effect of post-harvest drying process on chlorogenic acids, antioxidant activities and CIE-Lab color of Thai Arabica green coffee beans. Food Chem. 2022, 366, 130504. [Google Scholar] [CrossRef] [PubMed]
- Lobo, F.A.; Nascimento, M.A.; Domingues, J.R.; Falcao, D.Q.; Hernanz, D.; Heredia, F.J.; de Lima Araujo, K.G. Foam mat drying of Tommy Atkins mango: Effects of air temperature and concentrations of soy lecithin and carboxymethylcellulose on phenolic composition, mangiferin, and antioxidant capacity. Food Chem. 2017, 221, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.Y.; Xie, C.Z.; Mazhar, M.; Zhu, Y.; Zeng, H.Y.; Qin, L.K.; Zhu, Y. Comparative evaluation of drying methods on kinetics, biocompounds and antioxidant activity of Bacillus subtilis-fermented dehulled adlay. Dry. Technol. 2020, 38, 1505–1515. [Google Scholar] [CrossRef]
- Rico, D.; Villaverde, A.; Martinez-Villaluenga, C.; Gutierrez, A.L.; Caballero, P.A.; Ronda, F.; Penas, E.; Frias, J.; Martin Diana, A.B. Application of Autoclave Treatment for Development of a Natural Wheat Bran Antioxidant Ingredient. Foods 2020, 9, 781. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, Y.; Lagnika, C.; Li, D.; Liu, C.; Jiang, N.; Song, J.; Zhang, M. A comparative evaluation of nutritional properties, antioxidant capacity and physical characteristics of cabbage (Brassica oleracea var. Capitate var L.) subjected to different drying methods. Food Chem. 2020, 309, 124935. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, M.; Mujumdar, A.S.; Xu, J.; Wang, W. Influence of drying methods on the drying kinetics, bioactive compounds and flavor of solid-state fermented okara. Dry. Technol. 2019, 39, 644–654. [Google Scholar] [CrossRef]
- Srinivas, K.; Pandey, K.K. Effect of Heat Treatment on Color Changes, Dimensional Stability, and Mechanical Properties of Wood. J. Wood Chem. Technol. 2012, 32, 304–316. [Google Scholar] [CrossRef]











| Model Name | HAD | IR-HAD | IR-HAD + HAD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 55 °C | 65 °C | 75 °C | 55 °C | 65 °C | 75 °C | 55 °C | 65 °C | 75 °C | ||
| Two-Term | a | 0.5042 | 0.5069 | 0.5180 | 0.9650 | 0.9167 | 1.018 | 0.8539 | 0.8291 | 0.8373 |
| MR = aexp (−k × t) + bexp (−k1 × t) | k | 0.0016 | 0.0021 | 0.0027 | 0.0052 | 0.0044 | 0.0091 | 0.0017 | 0.0024 | 0.0048 |
| b | 0.5041 | 0.5068 | 0.5177 | 0.1164 | 0.2278 | 0.6357 | 0.8329 | 0.6127 | 0.3396 | |
| k1 | 0.0016 | 0.0021 | 0.0027 | 0.9228 | 0.0710 | 0.7537 | 0.6072 | 0.4640 | 0.7922 | |
| R2 | 0.9972 | 0.9987 | 0.9960 | 0.9940 | 0.9955 | 0.9791 | 0.9804 | 0.9796 | 0.9825 | |
| RMSE | 0.0198 | 0.0121 | 0.0222 | 0.0185 | 0.0154 | 0.0356 | 0.0355 | 0.0349 | 0.0298 | |
| SSE | 6.3 × 10−3 | 2.5 × 10−3 | 9.3 × 10−3 | 5.5 × 10−3 | 3.8 × 10−3 | 7.6 × 10−3 | 2.0 × 10−2 | 2.2 × 10−2 | 7.1 × 10−3 | |
| AIC | −118.85 | −144.87 | −157.16 | −206.99 | −176.85 | −58.05 | −132.09 | −138.22 | −79.09 | |
| BIC | −127.38 | −152.94 | −163.98 | −213.06 | −183.67 | −73.12 | −139.16 | −145.04 | −90.73 | |
| Page | k | 0.0011 | 0.0018 | 0.0010 | 0.0075 | 0.0062 | 0.0061 | 0.0110 | 0.0155 | 0.0174 |
| MR = exp (−k × tn) | n | 1.0626 | 1.0997 | 1.1493 | 0.9209 | 0.9741 | 1.0779 | 0.7310 | 0.7276 | 0.7800 |
| R2 | 0.9982 | 0.9987 | 0.9976 | 0.9953 | 0.9959 | 0.9962 | 0.9922 | 0.9908 | 0.9957 | |
| RMSE | 0.0146 | 0.0101 | 0.0180 | 0.0191 | 0.0187 | 0.0235 | 0.0230 | 0.0240 | 0.0161 | |
| SSE | 5.1 × 10−3 | 3.4 × 10−3 | 6.4 × 10−3 | 7.3 × 10−3 | 6.7 × 10−3 | 3.9 × 10−3 | 9.5 × 10−3 | 1.2 × 10−2 | 3.6 × 10−3 | |
| AIC | −130.05 | −146.62 | −171.80 | −205.54 | −170.79 | −77.96 | −154.31 | −157.97 | −97.83 | |
| BIC | −135.82 | −152.26 | −177.06 | −210.55 | −176.05 | −85.31 | −159.64 | −163.23 | −104.41 | |
| Logarithmic | a | 1.0752 | 1.0519 | 1.1001 | 0.9751 | 1.0048 | 1.0807 | 0.8401 | 0.8209 | 0.8373 |
| MR = aexp (−k × t) + b | k | 0.0015 | 0.0020 | 0.0021 | 0.0044 | 0.0047 | 0.0073 | 0.0018 | 0.0026 | 0.0047 |
| b | −0.0813 | −0.0441 | −0.1316 | −0.0183 | −0.0374 | −0.1166 | 0.0228 | 0.0225 | 0.0252 | |
| R2 | 0.9997 | 0.9997 | 0.9996 | 0.9952 | 0.9977 | 0.9993 | 0.9826 | 0.9812 | 0.9846 | |
| RMSE | 0.0065 | 0.0078 | 0.0074 | 0.0200 | 0.0142 | 0.0141 | 0.0335 | 0.0335 | 0.0236 | |
| SSE | 8.6 × 10−4 | 7.8 × 10−4 | 1.1 × 10−3 | 7.6 × 10−3 | 3.6 × 10−3 | 7.4 × 10−4 | 1.9 × 10−2 | 2.1 × 10−2 | 1.2 × 10−2 | |
| AIC | −156.83 | −169.76 | −207.52 | −201.68 | −181.44 | −91.01 | −136.66 | −142.64 | −77.84 | |
| BIC | −163.77 | −176.44 | −213.48 | −207.19 | −187.40 | −101.28 | −142.77 | −148.60 | −86.45 | |
| Henderson-Pabis | a | 1.011 | 1.008 | 1.025 | 0.9645 | 0.9332 | 1.018 | 0.8539 | 0.8291 | 0.8373 |
| MR = aexp (−k × t) | k | 0.0017 | 0.0022 | 0.0026 | 0.0052 | 0.0045 | 0.0091 | 0.0016 | 0.0024 | 0.0048 |
| R2 | 0.9973 | 0.9984 | 0.9964 | 0.9947 | 0.9956 | 0.9843 | 0.9826 | 0.9816 | 0.9860 | |
| RMSE | 0.0174 | 0.0138 | 0.0211 | 0.0175 | 0.0152 | 0.0294 | 0.0335 | 0.0331 | 0.0266 | |
| SSE | 6.3 × 10−3 | 2.5 × 10−3 | 9.3 × 10−3 | 5.5 × 10−3 | 4.2 × 10−3 | 7.6 × 10−3 | 2.0 × 10−2 | 2.2 × 10−2 | 7.1 × 10−3 | |
| AIC | −126.46 | −152.16 | −163.58 | −212.90 | −181.07 | −70.62 | −138.68 | −144.64 | −89.00 | |
| BIC | −132.23 | −157.79 | −168.83 | −217.91 | −186.32 | −77.97 | −144.01 | −149.89 | −95.58 | |
| Drying Methods | Temperature | Deff | Ea | Color Parameters | SR | WBC | WHC | TPC | TFC | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (10−7 m2∙s−1) | kJ/mol | L* | a* | b* | ∆E | (%) | (g/g) | (mg GAE/g DW) | (mg RE/g DW) | ||
| Control | - | - | 80.63 ± 0.27 a | 0.93 ± 0.10 d | 9.19 ± 0.32 j | - | 16.26 ± 0.56 g | 8.35 ± 0.68 a | 7.87 ± 0.13 a | 40.43 ± 2.66 cde | 119.32 ± 3.04 c | |
| HAD | 55 | 0.61 | 26.21 | 79.08 ± 0.13 ab | 1.08 ± 0.13 d | 14.06 ± 0.25 cde | 5.02 ± 0.04 e | 26.38 ± 1.30 cde | 4.73 ± 0.17 f | 6.73 ± 0.30 bcd | 26.91 ± 0.40 g | 100.43 ± 1.58 e |
| 65 | 0.82 | 73.96 ± 0.78 cd | 1.27 ± 0.29 d | 13.21 ± 0.68 de | 8.21 ± 0.64 cd | 26.85 ± 2.33 bcd | 5.11 ± 0.03 ef | 6.98 ± 0.65 abc | 44.51 ± 0.28 bc | 110.99 ± 0.97 d | ||
| 75 | 1.07 | 64.91 ± 3.07 e | 1.96 ± 0.54 c | 13.69 ± 1.04 cde | 16.44 ± 2.93 b | 31.04 ± 2.60 ab | 4.74 ± 0.64 f | 6.02 ± 0.28 cde | 35.07 ± 3.79 ef | 118.81 ± 1.09 c | ||
| IR-HAD | 55 | 1.01 | 60.76 | 78.18 ± 0.47 ab | 1.59 ± 0.09 cd | 14.98 ± 0.17 bc | 6.46 ± 0.14 de | 26.40 ± 1.27 cde | 6.06 ± 0.57 cde | 6.45 ± 0.12 bcd | 45.69 ± 1.23 bc | 121.45 ± 1.75 c |
| 65 | 1.67 | 76.56 ± 0.48 bc | 1.65 ± 0.06 cd | 14.59 ± 0.26 bc | 6.28 ± 0.30 de | 22.19 ± 4.26 ef | 6.83 ± 0.09 bc | 7.69 ± 0.44 ab | 52.31 ± 2.49 a | 133.49 ± 2.91 a | ||
| 75 | 3.64 | 66.71 ± 2.83 e | 3.42 ± 1.24 b | 15.77 ± 0.70 ab | 14.73 ± 2.67 b | 21.62 ± 1.65 f | 6.50 ± 0.45 bc | 6.97 ± 0.35 abc | 34.55 ± 3.73 ef | 114.68 ± 0.61 cd | ||
| IR-HAD + HAD | 55 | 0.48 | 52.44 | 79.39 ± 1.11 ab | 0.67 ± 0.04 d | 11.84 ± 0.30 f | 5.00 ± 0.28 e | 28.31 ± 1.90 bc | 5.44 ± 0.46 def | 5.31 ± 0.33 e | 42.15 ± 0.11 de | 86.26 ± 0.49 f |
| 65 | 0.81 | 75.98 ± 2.44 c | 1.46 ± 0.07 cd | 14.29 ± 0.33 cd | 6.84 ± 1.50 de | 23.32 ± 1.85 def | 7.07 ± 0.39 b | 5.70 ± 0.23 de | 49.91 ± 2.38 ab | 117.35 ± 2.43 c | ||
| 75 | 1.45 | 71.94 ± 1.34 d | 2.30 ± 1.63 bc | 12.85 ± 1.46 ef | 9.93 ± 1.16 c | 34.10 ± 0.72 a | 5.84 ± 0.45 cde | 5.58 ± 0.48 e | 44.71 ± 0.79 cd | 115.46 ± 4.37 cd | ||
| No | Compounds | Formula | Content (μg/kg) | |||
|---|---|---|---|---|---|---|
| Control | HAD | IR-HAD | IR-HAD + HAD | |||
| Terpenes | ||||||
| 1 | 1-Isopropyl-4-methylenebicyclo[3.1.0]hexane | C10H16 | 126.38 | 10.81 | 22.15 | 12.67 |
| 2 | D-Limonene | C10H16 | 28.49 | 5.43 | - | - |
| 3 | β-cis-Ocimene | C10H16 | 14.47 | - | - | 6.06 |
| 4 | Ylangene | C15H24 | - | - | 9.15 | 5.19 |
| 5 | trans-β-Bergamotene | C15H24 | 39.52 | 5.23 | 10.68 | 5.76 |
| 6 | β-Caryophyllene | C15H24 | 144.34 | 40.17 | 45.29 | 45.52 |
| 7 | (E)-β-Famesene | C15H24 | 227.11 | 21.21 | 37.44 | 26.74 |
| 8 | β-Bisabolene | C15H24 | 686.14 | 189.02 | 287.07 | 232.92 |
| 9 | γ-Muurolene | C15H24 | 164.78 | 124.97 | 321.08 | 190.58 |
| 10 | α-Zingiberene | C15H24 | 391.72 | 294.86 | 632.19 | 545.52 |
| 11 | Bisabolene | C15H24 | 99.51 | 43.45 | 67.44 | 64.84 |
| 12 | (Z,E)-α-Farnesene | C15H24 | 178.91 | 89.74 | 166.21 | 136.33 |
| 13 | α-Farnesene | C15H24 | 178.68 | 53.62 | 87.36 | 73.14 |
| 14 | β-Sesquiphellandrene | C15H24 | 555.49 | 475.34 | 824.78 | 965.02 |
| 15 | α-Curcumene | C15H22 | 172.97 | 101.50 | 153.41 | 131.91 |
| 16 | Eucalyptol | C10H18O | 330.23 | 16.20 | 28.33 | 20.16 |
| 17 | (1R,2R,5S)-5-Isopropyl-2-methylbicyclo[3.1.0]hexan-2-ol | C10H18O | 191.12 | 7.61 | 14.00 | 9.16 |
| 18 | Linalool | C10H18O | 108.97 | 6.27 | 8.11 | 7.77 |
| 19 | Terpinen-4-ol | C10H18O | - | 9.39 | 10.38 | 5.91 |
| 20 | Isoborneol | C10H18O | 1553.76 | 174.71 | 246.03 | 142.13 |
| 21 | endo-Borneol | C10H18O | 1618.74 | - | - | - |
| 22 | Nerolidol | C15H26O | - | 19.73 | - | 36.69 |
| 23 | 7-epi-γ-Eudesmol | C15H26O | 70.26 | 8.61 | 58.67 | 34.52 |
| 24 | Eudesm-7(11)-en-4-ol | C15H26O | 158.83 | 122.90 | 100.63 | 250.93 |
| 25 | Carveol | C10H16O | 91.97 | 19.39 | 26.76 | 8.98 |
| Ketones | ||||||
| 26 | 6-Methyl-5-heptene-2-one | C8H14O | 15.14 | - | - | - |
| 27 | Bicyclo[3.1.1]hept-2-en-6-one, 2,7,7-trimethyl- | C10H14O | 95.26 | - | - | 36.25 |
| 28 | Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1S)- | C10H16O | 1472.76 | 35.48 | 72.71 | - |
| 29 | D-Carvone | C10H14O | 101.51 | 11.90 | 16.50 | 11.29 |
| 30 | l-Verbenone | C10H14O | 128.40 | 3.02 | - | 5.11 |
| Esters | ||||||
| 31 | trans-Chrysanthenyl acetate | C12H18O2 | 35.70 | 2.68 | - | 2.13 |
| 32 | Bornyl acetate | C12H18O2 | 174.18 | 32.97 | 68.60 | 49.22 |
| 33 | Methyl caprate | C11H22O2 | 88.94 | 11.06 | 14.10 | 20.13 |
| 34 | Benzyl pentanoate | C12H16O2 | 265.17 | 38.33 | 49.94 | 26.08 |
| 35 | Benzyl isovalerate | C12H16O2 | 46.80 | 7.45 | 13.00 | 10.07 |
| Hydrocarbon | ||||||
| 36 | p-Xylene | C8H10 | - | 4.12 | - | - |
| 37 | Undecane | C11H24 | 21.71 | 6.33 | - | - |
| 38 | 1,3,5,7-Cyclooctatetraene | C8H8 | 38.88 | 19.47 | 41.59 | 17.19 |
| 39 | 1-Pentadecene | C15H30 | - | 4.27 | 8.09 | 6.70 |
| Aldehydes | ||||||
| 40 | Benzaldehyde | C7H6O | 92.38 | 22.21 | 37.40 | 26.96 |
| 41 | 3-Hydroxybenzaldehyde | C7H6O | 56.04 | 23.45 | 41.17 | 37.52 |
| 42 | 4-iPr-Benzaldehyde | C10H12O | 4.72 | 6.68 | ||
| 43 | Furfural | C5H4O2 | - | 3.87 | 23.85 | 2.91 |
| Alcohols | ||||||
| 44 | 2,4-Dimethyl-4-octanol | C10H22O | - | 19.15 | - | 5.66 |
| 45 | 3-Methyl-3-cyclohexen-1-ol | C7H12O | - | 29.07 | 36.29 | 4.87 |
| 46 | Benzyl alcohol | C7H8O | 25.58 | 11.92 | 9.95 | 6.18 |
| Phenols | ||||||
| 47 | Butylated Hydroxytoluene | C15H24O | - | 5.97 | 11.02 | - |
| Furans | ||||||
| 48 | Dihydro-β-agarofuran | C15H26O | 57.27 | 13.62 | 14.37 | 15.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Wu, M.; Wang, Y.; Wei, W.; Sun, D.; Li, D.; Zheng, Z.; Gao, F. Effect of Combined Infrared and Hot Air Drying Strategies on the Quality of Chrysanthemum (Chrysanthemum morifolium Ramat.) Cakes: Drying Behavior, Aroma Profiles and Phenolic Compounds. Foods 2022, 11, 2240. https://doi.org/10.3390/foods11152240
Xu H, Wu M, Wang Y, Wei W, Sun D, Li D, Zheng Z, Gao F. Effect of Combined Infrared and Hot Air Drying Strategies on the Quality of Chrysanthemum (Chrysanthemum morifolium Ramat.) Cakes: Drying Behavior, Aroma Profiles and Phenolic Compounds. Foods. 2022; 11(15):2240. https://doi.org/10.3390/foods11152240
Chicago/Turabian StyleXu, Huihuang, Min Wu, Yong Wang, Wenguang Wei, Dongyu Sun, Dong Li, Zhian Zheng, and Fei Gao. 2022. "Effect of Combined Infrared and Hot Air Drying Strategies on the Quality of Chrysanthemum (Chrysanthemum morifolium Ramat.) Cakes: Drying Behavior, Aroma Profiles and Phenolic Compounds" Foods 11, no. 15: 2240. https://doi.org/10.3390/foods11152240