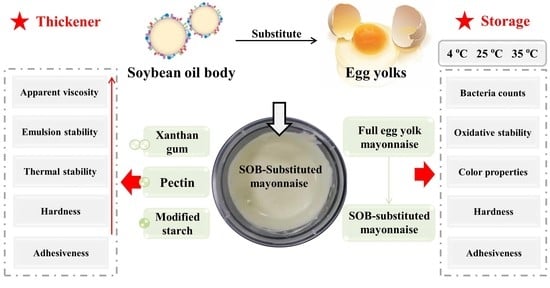
Physicochemical Properties, Stability and Texture of Soybean-Oil-Body-Substituted Low-Fat Mayonnaise: Effects of Thickeners and Storage Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SOB
2.3. Preparation of Mayonnaise Samples
2.4. Apparent Viscosity
2.5. Emulsion Stability
2.6. Thermal Stability
2.7. Texture
2.8. Microstructure
2.9. Total Bacteria Count
2.10. Peroxide Value (PV)
2.11. Thiobarbituric Acid Reactive Substance (TBARS)
2.12. PH
2.13. Color Properties
2.14. Statistical Analysis
3. Results
3.1. Apparent Viscosity of SOB-Substituted Mayonnaise
3.2. Emulsion Stability and Thermal Stability of SOB-Substituted Mayonnaise
3.3. Texture of SOB-Substituted Mayonnaise
3.4. Microstructure of SOB-Substituted Mayonnaise
3.5. Total Bacteria Counts in Mayonnaise during Storage
3.6. Oxidative Stability of Mayonnaise during Storage
3.7. pH of Mayonnaise during Storage
3.8. Hardness and Adhesiveness of Mayonnaise during Storage
3.9. Color Properties of Mayonnaise during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, C.; Liu, R.; Liang, B.; Wu, T.; Sui, W.; Zhang, M. Microparticulated whey protein-pectin complex: A texture-controllable gel for low-fat mayonnaise. Food Res. Int. 2018, 108, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Roy, S.; Devra, A.; Dhiman, A.; Prabhakar, P.K. Ultrasonication of mayonnaise formulated with xanthan and guar gums: Rheological modeling, effects on optical properties and emulsion stability. LWT 2021, 149, 111632. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Yu, H.; Mu, S.; Li, H.; Liu, X.; Zhang, M.; Jiang, Z.; Hou, J. Biological activities and in vitro digestion characteristics of glycosylated α-lactalbumin prepared by microwave heating: Impacts of ultrasonication. LWT 2022, 158, 113141. [Google Scholar] [CrossRef]
- Alizadeh, L.; Abdolmaleki, K.; Nayebzadeh, K.; Shahin, R. Effects of tocopherol, rosemary essential oil and Ferulago angulata extract on oxidative stability of mayonnaise during its shelf life: A comparative study. Food Chem. 2019, 285, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, J.; Zhao, X.; Sun, R.; Sun, C.; Hou, D.; Zhang, X.; Jiang, L.; Hou, J.; Jiang, Z. Oil bodies extracted from high-oil soybeans (Glycine max) exhibited higher oxidative and physical stability than oil bodies from high-protein soybeans. Food Funct. 2022, 13, 3271–3282. [Google Scholar] [CrossRef]
- Mirzanajafi-Zanjani, M.; Yousefi, M.; Ehsani, A. Challenges and approaches for production of a healthy and functional mayonnaise sauce. Food Sci. Nutr. 2019, 7, 2471–2484. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.; Xu, C.; Liu, Z.; Gu, L.; Ma, J.; Jiang, L.; Jiang, Z.; Hou, J. Effects of soybean oil body as a milk fat substitute on ice cream: Physicochemical, sensory and digestive properties. Foods 2022, 11, 1504. [Google Scholar] [CrossRef]
- Zaaboul, F.; Zhao, Q.; Xu, Y.; Liu, Y. Soybean oil bodies: A review on composition, properties, food applications, and future research aspects. Food Hydrocoll. 2022, 124, 107296. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, T.; Gantumur, M.-A.; Qayum, A.; Bilawal, A.; Jiang, Z.; Wang, L. Non-covalent interaction and digestive characteristics between α-lactalbumin and safflower yellow: Impacts of microwave heating temperature. LWT 2022, 159, 113206. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I. Advances in the Design and Production of Reduced-Fat and Reduced-Cholesterol Salad Dressing and Mayonnaise: A Review. Food Bioprocess Technol. 2013, 6, 648–670. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Emadzadeh, B.; Kadkhodaee, R. Effects of pectin and xanthan gum on induced-flocculation phenomenon in an acidic model emulsion system. J. Dispers. Sci. Technol. 2019, 40, 256–263. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, R.; Zhao, J.; Liu, Z.; Wang, M.; Wang, K.; Jiang, L.; Hou, J.; Jiang, Z. Enzymatic activity and stability of soybean oil body emulsions recovered under neutral and alkaline conditions: Impacts of thermal treatments. LWT 2022, 153, 112545. [Google Scholar] [CrossRef]
- León, O.; Soto, D.; López, D.; Muñoz-Bonilla, A.; Fernández-García, M. Fat-replacer properties of oxidized cassava starch using hydrogen peroxide/sodium bicarbonate redox system in mayonnaise formulation and its stability. Starch-Stärke 2019, 71, 1900112. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, Y.; Li, J.; Wang, K.; Ma, C.; Sun, D.; Hussain, M.A.; Qayum, A.; Hou, J. Consecutive pH-shift and ultrasound treatment modify the physicochemical properties of whey protein isolate. Int. Dairy J. 2022, 127, 105211. [Google Scholar] [CrossRef]
- Li, J.; Fu, J.; Ma, Y.; He, Y.; Fu, R.; Qayum, A.; Jiang, Z.; Wang, L. Low temperature extrusion promotes transglutaminase cross-linking of whey protein isolate and enhances its emulsifying properties and water holding capacity. Food Hydrocoll. 2022, 125, 107410. [Google Scholar] [CrossRef]
- Nikzade, V.; Tehrani, M.M.; Saadatmand-Tarzjan, M. Optimization of low-cholesterol–low-fat mayonnaise formulation: Effect of using soy milk and some stabilizer by a mixture design approach. Food Hydrocoll. 2012, 28, 344–352. [Google Scholar] [CrossRef]
- Liu, H.; Xu, X.M.; Guo, S.D. Rheological, texture and sensory properties of low-fat mayonnaise with different fat mimetics. LWT Food Sci. Technol. 2007, 40, 946–954. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Zhao, J.; Yu, R.; Altaf Hussain, M.; Qayum, A.; Jiang, Z.; Qu, B. Glycosylated whey protein isolate enhances digestion behaviors and stabilities of conjugated linoleic acid oil in water emulsions. Food Chem. 2022, 383, 132402. [Google Scholar] [CrossRef]
- Santipanichwong, R.; Suphantharika, M. Carotenoids as colorants in reduced-fat mayonnaise containing spent brewer’s yeast β-glucan as a fat replacer. Food Hydrocoll. 2007, 21, 565–574. [Google Scholar] [CrossRef]
- Liu, X.; Guo, J.; Wan, Z.-L.; Liu, Y.-Y.; Ruan, Q.-J.; Yang, X.-Q. Wheat gluten-stabilized high internal phase emulsions as mayonnaise replacers. Food Hydrocoll. 2018, 77, 168–175. [Google Scholar] [CrossRef]
- Yang, X.; Gong, T.; Lu, Y.-h.; Li, A.; Sun, L.; Guo, Y. Compatibility of sodium alginate and konjac glucomannan and their applications in fabricating low-fat mayonnaise-like emulsion gels. Carbohydr. Polym. 2020, 229, 115468. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Casas, K.-G.; Laguado-Escobar, D.-A.; Narváez-Cuenca, C.-E. Using a mixture of hydrocolloids to mimic texture and rheological properties of a massive consumption food product. J. Food Process. Preserv. 2022, 46, e16440. [Google Scholar] [CrossRef]
- Golchoobi, L.; Alimi, M.; Shokoohi, S.; Yousefi, H. Interaction between Nanofibrillated Cellulose with Guar Gum and Carboxy Methyl Cellulose in Low-Fat Mayonnaise. J. Texture Stud. 2016, 47, 403–412. [Google Scholar] [CrossRef]
- Ketenoglu, O.; Mert, B.; Tekin, A. Effects of Microfluidized Dietary Fibers on Stability Properties of Emulsions. J. Texture Stud. 2014, 45, 295–306. [Google Scholar] [CrossRef]
- Sikora, M.; Badrie, N.; Deisingh, A.K.; Kowalski, S. Sauces and Dressings: A Review of Properties and Applications. Crit. Rev. Food Sci. Nutr. 2008, 48, 50–77. [Google Scholar] [CrossRef]
- Abedinzadeh, S.; Torbati, M.; Azadmard-Damirchi, S. Some Qualitative and Rheological Properties of Virgin Olive Oil- Apple Vinegar Salad Dressing Stabilized With Xanthan Gum. Adv. Pharm. Bull. 2016, 6, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Cai, X.; Ding, L.; Wang, S. Effect of pH, ionic strength, chitosan deacetylation on the stability and rheological properties of O/W emulsions formulated with chitosan/casein complexes. Food Hydrocoll. 2021, 111, 106211. [Google Scholar] [CrossRef]
- Raikos, V.; McDonagh, A.; Ranawana, V.; Duthie, G. Processed beetroot (Beta vulgaris L.) as a natural antioxidant in mayonnaise: Effects on physical stability, texture and sensory attributes. Food Sci. Hum. Wellness 2016, 5, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Alimi, M.; Mizani, M.; Naderi, G.; Shokoohi, S. Effect of inulin formulation on the microstructure and viscoelastic properties of low-fat mayonnaise containing modified starch. J. Appl. Polym. Sci. 2013, 130, 801–809. [Google Scholar] [CrossRef]
- Raikos, V.; Hayes, H.; Ni, H. Aquafaba from commercially canned chickpeas as potential egg replacer for the development of vegan mayonnaise: Recipe optimisation and storage stability. Int. J. Food Sci. Technol. 2020, 55, 1935–1942. [Google Scholar] [CrossRef]
- Sukhotu, R.; Shi, X.; Hu, Q.; Nishinari, K.; Fang, Y.; Guo, S. Aggregation behaviour and stability of maize germ oil body suspension. Food Chem. 2014, 164, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, J.; Sun, R.; Wang, M.; Wang, K.; Li, Y.; Shang, H.; Hou, J.; Jiang, Z. Lactobacillus plantarum 23-1 improves intestinal inflammation and barrier function through the TLR4/NF-κB signaling pathway in obese mice. Food Funct. 2022, 13, 5971–5986. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, X.; Wang, W.; Gu, L.; Hu, C.; Sun, H.; Xu, C.; Hou, J.; Jiang, Z. Lactobacillus paracasei 24 Attenuates Lipid Accumulation in High-Fat Diet-Induced Obese Mice by Regulating the Gut Microbiota. J. Agric. Food Chem. 2022, 70, 4631–4643. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, F.; Xu, C.; Liu, Z.; Ma, J.; Gu, L.; Jiang, Z.; Hou, J. Lactobacillus plantarum 69-2 combined with galacto-oligosaccharides alleviates d-galactose-induced aging by regulating the AMPK/SIRT1 signaling pathway and gut microbiota in mice. J. Agric. Food Chem. 2021, 69, 2745–2757. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, C.; Zhou, X.; Zhang, L.; Gu, L.; Liu, Z.; Ma, J.; Hou, J.; Jiang, Z. Lactobacillus plantarum Combined with Galactooligosaccharides Supplement: A Neuroprotective Regimen Against Neurodegeneration and Memory Impairment by Regulating Short-Chain Fatty Acids and the c-Jun N-Terminal Kinase Signaling Pathway in Mice. J. Agric. Food Chem. 2022, 70, 8619–8630. [Google Scholar] [CrossRef]
- Xu, C.; Fu, Y.; Liu, F.; Liu, Z.; Ma, J.; Jiang, R.; Song, C.; Jiang, Z.; Hou, J. Purification and antimicrobial mechanism of a novel bacteriocin produced by Lactobacillus rhamnosus 1.0320. LWT 2021, 137, 110338. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, K.; Zhao, J.; Sun, R.; Shang, H.; Sun, C.; Liu, L.; Hou, J.; Jiang, Z. Physical and oxidative stability of astaxanthin microcapsules prepared with liposomes. J. Sci. Food Agric. 2022, 102, 4909–4917. [Google Scholar] [CrossRef]
- Fisk, I.D.; White, D.A.; Lad, M.; Gray, D.A. Oxidative stability of sunflower oil bodies. Eur. J. Lipid Sci. Technol. 2008, 110, 962–968. [Google Scholar] [CrossRef]
- Fisk, I.D.; Gray, D.A. Soybean (Glycine max) Oil Bodies and Their Associated Phytochemicals. J. Food Sci. 2011, 76, C1349–C1354. [Google Scholar] [CrossRef] [Green Version]
- Yolmeh, M.; Najafi, M.B.H.; Farhoosh, R.; Salehi, F. Modeling of antibacterial activity of annatto dye on Escherichia coli in mayonnaise. Food Biosci. 2014, 8, 8–13. [Google Scholar] [CrossRef]
- Shaygannia, S.; Eshaghi, M.R.; Fazel, M.; Hashemiravan, M. The Effect of Microencapsulation of Phenolic Compounds from Lemon Waste by Persian and Basil Seed Gums on the Chemical and Microbiological Properties of Mayonnaise. Prev. Nutr. Food Sci. 2021, 26, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cui, C.; Wang, Q.; Sun, C.; Jiang, L.; Hou, J. Effect of pH on physicochemical properties of oil bodies from different oil crops. J. Food Sci. Technol. 2019, 56, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Hakimian, F.; Emamifar, A.; Karami, M. Evaluation of microbial and physicochemical properties of mayonnaise containing zinc oxide nanoparticles. LWT 2022, 163, 113517. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Lee, J.-H. Effects of Hydrolyzed Rapeseed Cake Extract on the Quality Characteristics of Mayonnaise Dressing. J. Food Sci. 2017, 82, 2847–2856. [Google Scholar] [CrossRef]
- Park, J.J.; Olawuyi, I.F.; Lee, W.Y. Characteristics of low-fat mayonnaise using different modified arrowroot starches as fat replacer. Int. J. Biol. Macromol. 2020, 153, 215–223. [Google Scholar] [CrossRef]





| Stabilizer | Hardness (g) | Adhesiveness (g·sec) | Springiness | Cohesiveness |
|---|---|---|---|---|
| 1 none | 3 6.56 ± 0.16 f | 68.52 ± 0.59 f | 0.41 ± 0.04 a | 0.32 ± 0.03 a |
| xanthan gum | 7.25 ± 0.08 c | 73.36 ± 0.57 c | 0.43 ± 0.04 a | 0.33 ± 0.04 a |
| pectin | 7.02 ± 0.13 d | 71.89 ± 0.54 d | 0.39 ± 0.06 a | 0.34 ± 0.02 a |
| modified starch | 6.97 ± 0.15 e | 71.60 ± 0.48 e | 0.44 ± 0.03 a | 0.35 ± 0.02 a |
| 2 compound 1 | 7.82 ± 0.06 b | 76.97 ± 0.22 b | 0.41 ± 0.02 a | 0.34 ± 0.02 a |
| compound 2 | 8.41 ± 0.13 a | 79.53 ± 0.47 a | 0.42 ± 0.05 a | 0.32 ± 0.03 a |
| compound 3 | 7.71 ± 0.12 b | 75.13 ± 0.34 b | 0.40 ± 0.03 a | 0.34 ± 0.04 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Hu, C.; Sun, H.; Zhao, J.; Xu, C.; Ma, Y.; Ma, J.; Jiang, L.; Hou, J. Physicochemical Properties, Stability and Texture of Soybean-Oil-Body-Substituted Low-Fat Mayonnaise: Effects of Thickeners and Storage Temperatures. Foods 2022, 11, 2201. https://doi.org/10.3390/foods11152201
Wang W, Hu C, Sun H, Zhao J, Xu C, Ma Y, Ma J, Jiang L, Hou J. Physicochemical Properties, Stability and Texture of Soybean-Oil-Body-Substituted Low-Fat Mayonnaise: Effects of Thickeners and Storage Temperatures. Foods. 2022; 11(15):2201. https://doi.org/10.3390/foods11152201
Chicago/Turabian StyleWang, Wan, Chuanbing Hu, Hong Sun, Jiale Zhao, Cong Xu, Yue Ma, Jiage Ma, Lianzhou Jiang, and Juncai Hou. 2022. "Physicochemical Properties, Stability and Texture of Soybean-Oil-Body-Substituted Low-Fat Mayonnaise: Effects of Thickeners and Storage Temperatures" Foods 11, no. 15: 2201. https://doi.org/10.3390/foods11152201