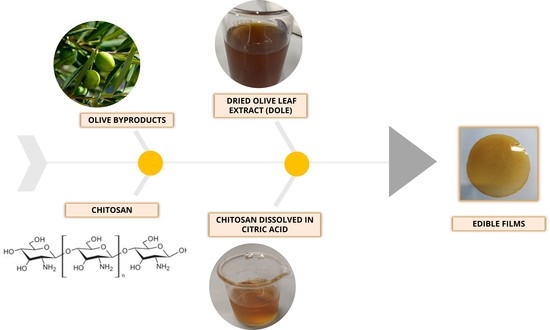
Edible Films Made of Dried Olive Leaf Extract and Chitosan: Characterization and Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. DOLE Preparation by Naviglio’s Principle
2.3. Total Polyphenols Content (TPC) Evaluation
2.4. Solubility of CH in CA
2.5. Preparation of Film Forming Solutions (FFSs) Containing DOLE
2.6. Zeta Potential and Particle Size Measurements
2.7. Films Preparation
2.8. Antioxidant Activity
2.9. In Vitro Oral Digestion
2.10. Film Mechanical Properties and Thickness
2.11. Bacterial Strains, Growth Conditions, and Antimicrobial Activity of DOLE-Based Films
2.12. DOLE Containing Film Capability to Contrast Bacterial Growth in Meat Samples
2.13. Statistical Analysis
3. Results
3.1. Olive Leaf Extract Preparation and Total Polyphenol Content (TPC)
3.2. Chitosan Solubility in Citric Acid
3.3. FFSs Characterization and Preparation of Films by Casting
3.4. Antioxidant Activity (AA) of DOLE-Containing Films over the Time
3.5. In Vitro Oral Digestion
3.6. Mechanical Properties
3.7. Antimicrobial Activity of Films Containing DOLE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elsabee, M.Z.; Abdou, E.S. Chitosan Based Edible Films and Coatings: A Review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-Based Nanomaterials: A State-of-the-Art Review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current Advancements in Chitosan-Based Film Production for Food Technology; A Review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial Activity of Chitosans and Chitosan Oligomers with Different Molecular Weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Melro, E.; Antunes, F.E.; da Silva, G.J.; Cruz, I.; Ramos, P.E.; Carvalho, F.; Alves, L. Chitosan Films in Food Applications. Tuning Film Properties by Changing Acidic Dissolution Conditions. Polymers 2021, 13, 1. [Google Scholar] [CrossRef]
- El Knidri, H.; Belabeed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the Degree of Acetylation and the Electrostatic Properties of Chitin and Chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef]
- Dotto, G.L.; Vieira, M.L.G.; Pinto, L.A.A. Use of Chitosan Solutions for the Microbiological Shelf Life Extension of Papaya Fruits during Storage at Room Temperature. LWT Food Sci. Technol. 2015, 64, 126–130. [Google Scholar] [CrossRef]
- Vimaladevi, S.; Panda, S.K.; Xavier, K.A.M.; Bindu, J. Packaging Performance of Organic Acid Incorporated Chitosan Films on Dried Anchovy (Stolephorus Indicus). Carbohydr. Polym. 2015, 127, 189–194. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-Zinc Oxide Nanoparticle Composite Coating for Active Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Del Hoyo-Gallego, S.; Pérez-Álvarez, L.; Gómez-Galván, F.; Lizundia, E.; Kuritka, I.; Sedlarik, V.; Laza, J.M.; Vila-Vilela, J.L. Construction of Antibacterial Poly(Ethylene Terephthalate) Films via Layer by Layer Assembly of Chitosan and Hyaluronic Acid. Carbohydr. Polym. 2016, 143, 35–43. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian Soft White Cheese Shelf Life Using a Novel Chitosan/Carboxymethyl Cellulose/Zinc Oxide Bionanocomposite Film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Guerrero-Beltrán, J.Á. Postharvest Quality of Peeled Prickly Pear Fruit Treated with Acetic Acid and Chitosan. Postharvest Biol. Technol. 2014, 92, 139–145. [Google Scholar] [CrossRef]
- Abdel-rahman, A.; Anyangwe, N.; Carlacci, L.; Casper, S.; Danam, R.P.; Enongene, E.; Erives, G.; Fabricant, D.; Gudi, R.; Hilmas, C.J.; et al. The Safety and Regulation of Natural Products Used as Foods and Food Ingredients. Toxicol. Sci. 2011, 123, 333–348. [Google Scholar] [CrossRef] [Green Version]
- Kiritsakis, K.; Goula, A.M.; Adamopoulos, K.G.; Gerasopoulos, D. Valorization of Olive Leaves: Spray Drying of Olive Leaf Extract. Waste Biomass Valoriz. 2018, 9, 619–633. [Google Scholar] [CrossRef]
- Espeso, J.; Isaza, A.; Lee, J.Y.; Sörensen, P.M.; Jurado, P.; de Avena-Bustillos, R.J.; Olaizola, M.; Arboleya, J.C. Olive Leaf Waste Management. Front. Sustain. Food Syst. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Özcan, M.M.; Matthäus, B. A Review: Benefit and Bioactive Properties of Olive (Olea Europaea L.) Leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential Use of Olive By-Products in Ruminant Feeding: A Review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Manzanares, P.; Ruiz, E.; Ballesteros, M.; Negro, M.J.; Gallego, F.J.; López-Linares, J.C.; Castro, E. Residual Biomass Potential in Olive Tree Cultivation and Olive Oil Industry in Spain: Valorization Proposal in a Biorefinery Context. Span. J. Agric. Res. 2017, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New Possibilities for the Valorization of Olive Oil By-Products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [Green Version]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea Europaea) Leaf Extract Effective in Patients with Stage-1 Hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef]
- Jemai, H.; Feki, A.E.L.; Sayadi, S. Antidiabetic and Antioxidant Effects of Hydroxytyrosol and Oleuropein from Olive Leaves in Alloxan-Diabetic Rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef]
- Khalatbary, A.R.; Zarrinjoei, G.R. Anti-Inflammatory Effect of Oleuropein in Experimental Rat Spinal Cord Trauma. Iran. Red Crescent Med. J. 2012, 14, 229–234. [Google Scholar]
- Sabry, O.M.M. Review: Beneficial Health Effects of Olive Leaves Extracts. J. Nat. Sci. Res. 2014, 4, 19. [Google Scholar]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant Activity of Phenolics Extracted from Olea Europaea L. Leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Andrikopoulos, N.; Kaliora, A.; Assimopoulou, A.; Papageorgiou, V. Inhibitory Activity of Minor Polyphenolic and Nonpolyphenolic Constituents of Olive Oil Against In Vitro Low-Density Lipoprotein Oxidation. J. Med. Food 2002, 5, 1–7. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Galli, C. Antioxidant and Other Biological Activities of Phenols from Olives and Olive Oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Sanz, M.T.; Blanco, B.; Beltrán, S.; Niknam, S.M. Freeze Dried Extract from Olive Leaves: Valorisation, Extraction Kinetics and Extract Characterization. Food Bioprod. Process. 2020, 124, 196–207. [Google Scholar] [CrossRef]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic Profile (HPLC-UV) of Olive Leaves According to Extraction Procedure and Assessment of Antibacterial Activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive Profile, Dehydration, Extraction and Application of the Bioactive Components of Olive Leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Pérez-Sánchez, A.; Herrero, M.; Ibañez, E.; Micol, V.; Zarrouk, M.; Segura-Carretero, A.; et al. Use of Advanced Techniques for the Extraction of Phenolic Compounds from Tunisian Olive Leaves: Phenolic Composition and Cytotoxicity against Human Breast Cancer Cells. Food Chem. Toxicol. 2012, 50, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Naviglio, D. Naviglio’s Principle and Presentation of an Innovative Solid-Liquid Extraction Technology: Extractor Naviglio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Dewanto, V.; Xianzhong, W.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and Characterization of Bioplastics from Grass Pea Flour Cast in the Presence of Microbial Transglutaminase. Coatings 2018, 8, 435. [Google Scholar] [CrossRef] [Green Version]
- Gaglione, R.; Cesaro, A.; Olmo, E.D.; Di Girolamo, R.; Tartaglione, L.; Pizzo, E.; Arciello, A. Cryptides Identified in Human Apolipoprotein B as New Weapons to Fight Antibiotic Resistance in Cystic Fibrosis Disease. Int. J. Mol. Sci. 2020, 21, 2049. [Google Scholar] [CrossRef] [Green Version]
- Abdalrazeq, M.; Jaradat, N.; Qadi, M.; Giosafatto, C.V.L.; Dell’olmo, E.; Gaglione, R.; Arciello, A.; Porta, R. Physicochemical and Antimicrobial Properties of Whey Protein-Based Films Functionalized with Palestinian Satureja Capitata Essential Oil. Coatings 2021, 11, 1364. [Google Scholar] [CrossRef]
- Pizarro, M.L.; Becerra, M.; Sayago, A.; Beltrán, M.; Beltrán, R. Comparison of Different Extraction Methods to Determine Phenolic Compounds in Virgin Olive Oil. Food Anal. Methods 2013, 6, 123–132. [Google Scholar] [CrossRef]
- Gimeno, E.; Castellote, A.I.; Lamuela-Raventós, R.M.; De la Torre, M.C.; López-Sabater, M.C. The Effects of Harvest and Extraction Methods on the Antioxidant Content (Phenolics, alfa -Tocopherol, and beta-Carotene) in Virgin Olive Oil. Food Chem. 2002, 78, 207–211. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Varriale, S.; Porta, R.; Naviglio, D.; Spennato, M.; Gardossi, L.; Giosafatto, C.V.L.; Pezzella, C. A Biorefinery Approach for the Conversion of Cynara Cardunculus Biomass to Active Films. Food Hydrocoll. 2022, 122, 107099. [Google Scholar] [CrossRef]
- Porta, R.; Mariniello, L.; di Pierro, P.; Sorrentino, A.; Giosafatto, C.V.L. Transglutaminase Crosslinked Pectinand Chitosan-Based Edible Films: A Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 223–238. [Google Scholar] [CrossRef]
- Yaseen, D.; Sabbah, M.; Al-Asmar, A.; Altamimi, M.; Famiglietti, M.; Giosafatto, C.V.L.; Mariniello, L. Functionality of Films from Nigella Sativa Defatted Seed Cake Proteins Plasticized with Grape Juice: Use in Wrapping Sweet Cherries. Coatings 2021, 11, 1383. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea Europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]







| Hours | Polyphenols (g GAE/L) |
|---|---|
| 2 | 4.4 ± 0.2 a |
| 4 | 4.6 ± 0.1 a |
| 6 | 5.5 ± 0.2 b |
| 24 | 6.6 ± 0.4 c |
| DOLE in FFSs % (v/v) | Z Potential (mV) | Mean Particle Size (nm) | PDI |
|---|---|---|---|
| 0 | 55.71 ± 4.61 a | 567.00 ± 19.33 a | 0.67 ± 0.02 a |
| 10 | 40.08 ± 3.20 c | 568.96 ± 31.40 a | 0.48 ± 0.01 b |
| 15 | 43.59 ± 2.51 c | 500.68 ± 28.30 b | 0.50 ± 0.03 b |
| 20 | 49.37 ± 5.42 b | 426.22 ± 49.05 c | 0.51 ± 0.05 b |
| 40 | 53.55 ± 5.61 a,b | 419.32 ± 73.02 c | 0.51 ± 0.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Famiglietti, M.; Savastano, A.; Gaglione, R.; Arciello, A.; Naviglio, D.; Mariniello, L. Edible Films Made of Dried Olive Leaf Extract and Chitosan: Characterization and Applications. Foods 2022, 11, 2078. https://doi.org/10.3390/foods11142078
Famiglietti M, Savastano A, Gaglione R, Arciello A, Naviglio D, Mariniello L. Edible Films Made of Dried Olive Leaf Extract and Chitosan: Characterization and Applications. Foods. 2022; 11(14):2078. https://doi.org/10.3390/foods11142078
Chicago/Turabian StyleFamiglietti, Michela, Alessandro Savastano, Rosa Gaglione, Angela Arciello, Daniele Naviglio, and Loredana Mariniello. 2022. "Edible Films Made of Dried Olive Leaf Extract and Chitosan: Characterization and Applications" Foods 11, no. 14: 2078. https://doi.org/10.3390/foods11142078