Inhibitory Mechanism of Advanced Glycation End-Product Formation by Avenanthramides Derived from Oats through Scavenging the Intermediates
Abstract
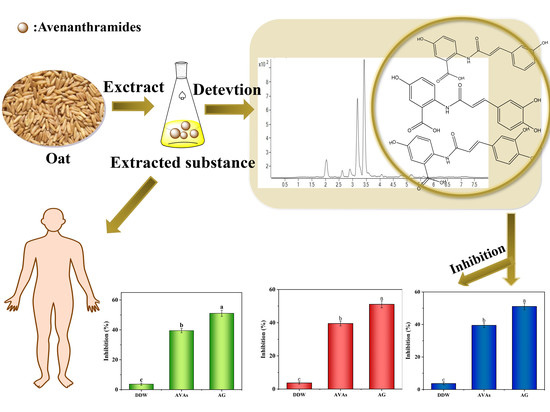
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Optimization of the Extraction of AVAs from Oats
2.3. HPLC Analysis of AVAs
2.4. Antioxidant Activity Analysis of DPPH
2.5. Antioxidant Activity Analysis of ABTS·+
2.6. Construction of the Glucose–Casein Simulation System
2.7. Determination of the Contents of Main AGEs
2.7.1. The HPLC–MS/MS Conditions for CML and CEL
2.7.2. The HPLC Conditions for the Determination of the PRL
2.8. Determination of AGEs Intermediates Captured by AVAs
2.9. Experiments on Scavenging Free Radicals by AVAs
2.10. Statistical Analysis
3. Results and Discussion
3.1. Optimization Results of AVA Extraction from Oats
3.2. Identification of the AVAs from Oat Extracts
3.3. Evaluation of the Antioxidant Activity of Oat Extracts
3.4. Inhibition of AVAs Extracted from Oats on AGEs in the Simulation System
| Target | Inhibitors | Inhibition (%) | References | |
|---|---|---|---|---|
| Source | Main Components | |||
| PRL | Highland barley whole grain | Phenolic compounds | 52.03 | [17] |
| CML | Standard substance | Phenolic compounds | 31.77 | [24] |
| PRL | Highland barley vinasse | Phenolic compounds | 49.22 | [17] |
| CML | Lotus seed waste | B-type phenolic acids | 29.40 | [25] |
| CML | Highland barley bran | Phenolic acids | 45.58 | [26] |
| AGEs | Millet | Phenolics | 68.3 | [3] |
| AGEs | Olive mill wastewater | Polyphenols | 43.0 | [27] |
| CML | Green coffee | Chlorogenic acids | 64.5 | [28] |
| AGEs | Standard substance | Capsaicin | 60.0 | [29] |
| CML | Standard substance | Catechins | - | [30] |
| CEL, CEL, PRL | Oats | AVAs | 39.5–51.2 | This Work |
3.5. Experimental Results of Capturing α-Dicarbonyl Compounds by AVAs
3.6. Clearance Rate Results
3.7. Scavenging of Radicals in the Glucose–Casein Simulation System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowotny, K.; Schröter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 47, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Huang, J.; Wang, M.; Ou, S. Effect of rosmarinic acid and carnosic acid on AGEs formation in vitro. Food Chem. 2017, 221, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Anis, M.A.; Sreerama, Y.N. Inhibition of protein glycoxidation and advanced glycation end-product formation by barnyard millet (Echinochloa frumentacea) phenolics. Food Chem. 2020, 315, 126265. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation. 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hull, G.L.J.; Woodside, J.V.; Ames, J.M.; Cuskelly, G.J. Nε-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012, 131, 170–174. [Google Scholar] [CrossRef]
- Liu, H.L.; Wu, D.; Zhou, K.; Wang, J.; Sun, B. Development and applications of molecularly imprinted polymers based on hydrophobic CdSe/ZnS quantum dots for optosensing of Nϵ-carboxymethyllysine in foods. Food Chem. 2016, 211, 34–40. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, S.; Chen, Y.; Zhang, R.; Zhou, M.; Wang, C.; Feng, N.; Wu, Q. Structure-activity relationship of procyanidins on advanced glycation end products formation and corresponding mechanisms. Food Chem. 2019, 272, 679–687. [Google Scholar] [CrossRef]
- Nguyen, S.; Pascariu, M.; Ghitescu, L. Early glycation products of endothelial plasma membrane proteins in experimental diabetes. Biochim Biophys Acta Mol. Basis Dis. 2006, 1762, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Westermann, S.; Brüggemann, D.A.; Olsen, K.; Skibsted, L.H. Light-induced formation of free radicals in cream cheese. Food Chem. 2009, 116, 974–981. [Google Scholar] [CrossRef]
- Hallfrisch, J.; Scholfield, D.J.; Behall, K.M. Diets containing soluble oat extracts reduce urinary malondialdehyde in moderately hypercholesterolemic men and women. J. Nutr. Biochem. 1997, 8, 497–501. [Google Scholar] [CrossRef]
- Wang, H.-C.; Hung, C.-H.; Hsu, J.-D.; Yang, M.-Y.; Wang, S.-J.; Wang, C.-J. Inhibitory effect of whole oat on aberrant crypt foci formation and colon tumor growth in ICR and BALB/c mice. J. Cereal Sci. 2011, 53, 73–77. [Google Scholar] [CrossRef]
- Yang, J.; Wang, P.; Wu, W.; Zhao, Y.; Idehen, E.; Sang, S. Steroidal Saponins in Oat Bran. J. Agric. Food Chem. 2016, 64, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Haddock, J.; Blumberg, J.B.; McKay, D.L.; Wei, X.; Dolnikowski, G.; Chen, C.Y.O. Identification of methylated metabolites of oat avenanthramides in human plasma using UHPLC QtoF-MS. Int. J. Food Sci. Nutr. 2018, 69, 377–383. [Google Scholar] [CrossRef]
- Pellegrini, G.G.; Morales, C.C.; Wallace, T.C.; Plotkin, L.I.; Bellido, T. Avenanthramides prevent osteoblast and osteocyte apoptosis and induce osteoclast apoptosis in vitro in an Nrf2-Independent Manner. Nutrients 2016, 8, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrelli, A.; Goitre, L.; Salzano, A.M.; Moglia, A.; Scaloni, A.; Retta, S.F. Biological activities, health benefits, and therapeutic properties of avenanthramides: From skin protection to prevention and treatment of cerebrovascular diseases. Oxid. Med. Cell. Longev. 2018, 2018, 6015351. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Liu, H. Corn silk extract inhibit the formation of Nε-carboxy methyl lysine by scavenging glyoxal/methyl glyoxal in a casein glucose-fatty acid model system. Food Chem. 2020, 309, 125708. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Liu, H.; Sun, B. Ultrahigh sensitivity nitrogen-doping carbon nanotubes-based metamaterial-free flexible terahertz sensing platform for insecticides detection. Food Chem. 2022, 133467. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Li, M.; Liu, H.; Sun, B. Temperature-responsive covalent organic framework encapsulated carbon dots-based sensing platform for pyrethroids detection via fluorescence response and smartphone readout. J. Agric. Food Chem. 2022, 70, 6059–6071. [Google Scholar] [CrossRef]
- Skoglund, M.; Peterson, D.M.; Andersson, R.; Nilsson, J.; Dimberg, L.H. Avenanthramide content and related enzyme activities in oats as affected by steeping and germination. J. Cereal Sci. 2008, 48, 294–303. [Google Scholar] [CrossRef]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Marfil, R.; Giménez, R.; Martínez, O.; Bouzas, P.R.; Rufián-Henares, J.A.; Mesías, M.; Cabrera-Vique, C. Determination of polyphenols, tocopherols, and antioxidant capacity in virgin argan oil (Argania spinosa, Skeels). Eur. J. Lipid Sci. Technol. 2011, 113, 886–893. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Van Der Fels-Klerx, H.J.; Van Boekel, M.A. Kinetics of Nε-(carboxymethyl) lysine formation in aqueous model systems of sugars and casein. Food Chem. 2016, 192, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Assar, S.H.; Moloney, C.; Lima, M.; Magee, R.; Ames, J.M. Determination of Nε-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino Acid. 2009, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Mildner-Szkudlarz, S.; Siger, A.; Szwengiel, A.; Przygoński, K.; Wojtowicz, E.; Zawirska-Wojtasiak, R. Phenolic compounds reduce formation of Nε-(carboxymethyl)lysine and pyrazines formed by Maillard reactions in a model bread system. Food Chem. 2017, 231, 175–184. [Google Scholar] [CrossRef]
- Feng, N.; Shen, Y.; Hu, C.; Tan, J.; Huang, Z.; Wang, C.; Guo, Z.; Wu, Q.; Xiao, J. Inhibition of advanced glycation end products in yogurt by lotus seedpod oligomeric procyanidin. Front. Nutr. 2021, 8, 781998. [Google Scholar] [CrossRef] [PubMed]
- Troise, A.D.; Fiore, A.; Colantuono, A.; Kokkinidou, S.; Peterson, D.G.; Fogliano, V. Effect of olive mill wastewater phenol compounds on reactive carbonyl species and Maillard reaction end-products in ultrahigh-temperature-treated milk. J. Agric. Food Chem. 2014, 62, 10092–10100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Zhang, D.; Liu, J.; Wang, J.; Wang, S.; Sun, B. Baijiu vinasse extract scavenges glyoxal and inhibits the formation of Nε-carboxymethyllysine in dairy food. Molecules 2019, 24, 1526. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, X.; Lin, T.; Yin, B.; Li, Q.; Wang, L.; Shao, J.; Yang, J. The level variation of Nε-(carboxymethyl)lysine is correlated with chlorogenic acids in Arabica, L. Coffee beans under different process conditions. Food Chem. 2021, 343, 128458. [Google Scholar] [CrossRef]
- Xu, P.; Yang, X.; Wang, Y. Inhibition of non-enzymatic glycation by capsaicin: Targeting AGE-induced diabetic complications. New J. Chem. 2021, 45, 16048–16058. [Google Scholar] [CrossRef]
- Zhu, Z.; Bassey, A.P.; Khan, I.A.; Huang, M.; Zhang, X. Inhibitory mechanism of catechins against advanced glycation end products of glycated myofibrillar protein through anti-aggregation and anti-oxidation. LWT 2021, 147, 111550. [Google Scholar] [CrossRef]
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: A comprehensive review. Food Res. Int. 2020, 130, 108933. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wise, M.L. Distributions of nutrients and avenanthramides within oat grain and effects on pearled kernel composition. Food Chem. 2020, 336, 127668. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-Y.; Li, S.; Tan, D.; Pan, M.-H.; Sang, S.; Ho, C.-T. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol. Nutr. Food Res. 2006, 50, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Shao, X.I.; Wang, L.; Huang, D.; Ho, C.-T.; Sang, S. Stilbene glucoside from polygonum multiflorum Thunb.: A novel natural inhibitor of advanced glycation end product formation by trapping of methylglyoxal. J. Agric. Food Chem. 2010, 58, 2239. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, P.; Zhang, Y.; Zhang, D.; Han, L.; Liu, H.; Sun, B. Inhibitory Mechanism of Advanced Glycation End-Product Formation by Avenanthramides Derived from Oats through Scavenging the Intermediates. Foods 2022, 11, 1813. https://doi.org/10.3390/foods11121813
Zhu P, Zhang Y, Zhang D, Han L, Liu H, Sun B. Inhibitory Mechanism of Advanced Glycation End-Product Formation by Avenanthramides Derived from Oats through Scavenging the Intermediates. Foods. 2022; 11(12):1813. https://doi.org/10.3390/foods11121813
Chicago/Turabian StyleZhu, Pei, Ying Zhang, Dianwei Zhang, Luxuan Han, Huilin Liu, and Baoguo Sun. 2022. "Inhibitory Mechanism of Advanced Glycation End-Product Formation by Avenanthramides Derived from Oats through Scavenging the Intermediates" Foods 11, no. 12: 1813. https://doi.org/10.3390/foods11121813