Novel Dual-Color Immunochromatographic Assay Based on Chrysanthemum-like Au@polydopamine and Colloidal Gold for Simultaneous Sensitive Detection of Paclobutrazol and Carbofuran in Fruits and Vegetables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Animal Immunization and Preparation of Antibody
2.4. ELISA Method
2.5. Antibody Affinity Evaluation
2.6. Antibody Specificity Evaluation
2.7. Preparation of AuNPs and AuNP–CAR mAb Probe
2.8. Preparation of AuNC@PDA and AuNC@PDA–PBZ mAb Probe
2.9. Assembly of the Dual-Color Immunochromatographic Test Strip
2.10. Assay of the Dual-Color Immunochromatographic Test Strip
2.11. Sample Preparation
2.12. Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS) Method
2.13. Recovery Experiment
3. Results and Discussion
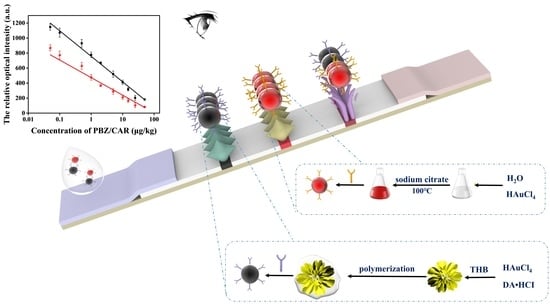
3.1. Principle of the Novel Dual-Color ICA
3.2. Characterization of Antigens and Antibodies
3.3. Characterization of AuNC@PDA and AuNPs
3.4. Optimization of the Novel Dual-Color ICA
3.5. Performance Evaluation of the Novel Dual-Color ICA
3.6. Recovery Rate Test
3.7. Accuracy Evaluation of ICA by LC–MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Opio, P.; Tomiyama, H.; Saito, T.; Ohkawa, K.; Ohara, H.; Kondo, S. Paclobutrazol elevates auxin and abscisic acid, reduces gibberellins and zeatin and modulates their transporter genes in Marubakaido apple rootstocks. Plant Physiol. Biochem. 2020, 155, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Gbadegesin, M.A.; Owumi, S.E.; Akinseye, V.; Odunola, O.A. Evaluation of hepatotoxicity and clastogenicity of carbofuran in male Wistar rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 65, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Kongphonprom, K.; Burakham, R. Determination of Carbamate Insecticides in Water, Fruit, and Vegetables by Ultrasound-Assisted Dispersive Liquid–Liquid Microextraction and High-Performance Liquid Chromatography. Anal. Lett. 2015, 49, 753–767. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y.; Xie, H.; Li, R.; Wei, J.; Liu, Y. Environmental behavior of paclobutrazol in soil and its toxicity on potato and taro plants. Environ. Sci. Pollut. Res. 2019, 26, 27385–27395. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Z.; Cui, S.; Xie, L.; Yu, J.; Tang, D.; Ma, X.; Mou, Y. Residue of Paclobutrazol and Its Regulatory Effects on the Secondary Metabolites of Ophiopogon japonicas. Molecules 2019, 24, 3504. [Google Scholar] [CrossRef]
- Hou, X.; Liu, L.; Wei, L.; Feng, D.; Lv, M.; Wang, X.; Yu, X.; Lu, Z.; Hou, Z. A Novel Analysis Method for Simultaneous Determination of 31 Pesticides by High-Performance Liquid Chromatography-Tandem Mass Spectrometry in Ginseng. J. Anal. Methods Chem. 2022, 2022, 4208243. [Google Scholar] [CrossRef]
- Ferracane, A.; Zoccali, M.; Cacciola, F.; Salerno, T.M.G.; Tranchida, P.Q.; Mondello, L. Determination of multi-pesticide residues in vegetable products using a “reduced-scale” Quechers method and flow-modulated comprehensive two-dimensional gas chromatography-triple quadrupole mass spectrometry. J. Chromatogr. A 2021, 1645, 462126. [Google Scholar] [CrossRef]
- Li, R.-X.; Li, M.-M.; Wang, T.; Wang, T.-L.; Chen, J.-Y.; Francis, F.; Fan, B.; Kong, Z.-Q.; Dai, X.-F. Screening of pesticide residues in Traditional Chinese Medicines using modified QuEChERS sample preparation procedure and LC-MS/MS analysis. J. Chromatogr. B 2020, 1152, 122224. [Google Scholar] [CrossRef]
- Chen, M.; Chen, L.; Pan, L.; Liu, R.; Guo, J.; Fan, M.; Wang, X.; Liu, H.; Liu, S. Simultaneous analysis of multiple pesticide residues in tobacco by magnetic carbon composite-based QuEChERS method and liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2022, 1668, 462913. [Google Scholar] [CrossRef]
- Mozzaquatro, J.d.O.; Cesar, I.A.; Barbosa Pinheiro, A.E.; Caldas, E.D. Pesticide residues analysis in passion fruit and its processed products by LC-MS/MS and GC-MS/MS: Method validation, processing factors and dietary risk assessment. Food Chem. 2022, 375, 131643. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, Y.H.; Huang, X.; Pan, J.R.; Jiao, B.N. Rapid Determination of Paclobutrazol in Fruits by QuEChERS Cleanup and Gas Chromatography-tandem Mass Spectrometry. Chin. J. Anal. Chem. 2011, 39, 1270–1273. [Google Scholar]
- Li, Y.; Li, J.; Huang, H.; Jian, D.; Shan, Y.; Wang, S.; Liu, F. Rapid quantitative detection for multiple antibiotics in honey using a quantum dot microsphere immunochromatographic strip. Food Control 2021, 130, 108256. [Google Scholar] [CrossRef]
- Song, C.; Liu, C.; Wu, S.; Li, H.; Guo, H.; Yang, B.; Qiu, S.; Li, J.; Liu, L.; Zeng, H.; et al. Development of a lateral flow colloidal gold immunoassay strip for the simultaneous detection of Shigella boydii and Escherichia coli O157:H7 in bread, milk and jelly samples. Food Control 2016, 59, 345–351. [Google Scholar] [CrossRef]
- Sun, J.; Wang, L.; Shao, J.; Yang, D.; Fu, X.; Sun, X. One-step time-resolved fluorescence microsphere immunochromatographic test strip for quantitative and simultaneous detection of DON and ZEN. Anal. Bioanal. Chem. 2021, 413, 6489–6502. [Google Scholar] [CrossRef]
- Xing, C.; Dong, X.; Xu, T.; Yuan, J.; Yan, W.; Sui, X.; Zhao, X. Analysis of multiple mycotoxins-contaminated wheat by a smart analysis platform. Anal. Biochem. 2020, 610, 113928. [Google Scholar] [CrossRef]
- Xing, C.; Liu, L.; Song, S.; Feng, M.; Kuang, H.; Xu, C. Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosens. Bioelectron. 2015, 66, 445–453. [Google Scholar] [CrossRef]
- Han, M.; Gong, L.; Wang, J.; Zhang, X.; Jin, Y.; Zhao, R.; Yang, C.; He, L.; Feng, X.; Chen, Y. An octuplex lateral flow immunoassay for rapid detection of antibiotic residues, aflatoxin M1 and melamine in milk. Sens. Actuators B Chem. 2019, 292, 94–104. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Y.; Liu, L.; Kuang, H.; Li, A.; Xu, C. Multiplex lateral flow immunoassay for five antibiotics detection based on gold nanoparticle aggregations. RSC Adv. 2016, 6, 7798–7805. [Google Scholar] [CrossRef]
- Qin, P.; Xu, J.; Yao, L.; Wu, Q.; Yan, C.; Lu, J.; Yao, B.; Liu, G.; Chen, W. Simultaneous and accurate visual identification of chicken, duck and pork components with the molecular amplification integrated lateral flow strip. Food Chem. 2020, 339, 127891. [Google Scholar] [CrossRef]
- Ni, T.; Peng, D.; Wang, Y.; Pan, Y.; Xie, S.; Chen, D.; Wang, Y.; Tao, Y.; Yuan, Z. Development of a broad-spectrum monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for the multi-residue detection of avermectins in edible animal tissues and milk. Food Chem. 2019, 286, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Liu, L.; Hao, C.; Xu, X.; Song, S.; Kuang, H.; Xu, C. Rapid and Sensitive Immunochromatographic Method-Based Monoclonal Antibody for the Quantitative Detection of Metalaxyl in Tobacco. ACS Omega 2020, 5, 18168–18175. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Lv, X.; Zhang, G.; Xiong, Z.; Lai, W.; Peng, J. Highly Specific Anti-tylosin Monoclonal Antibody and Its Application in the Quantum Dot Bead-Based Immunochromatographic Assay. Food Anal. Methods 2020, 13, 2258–2268. [Google Scholar] [CrossRef]
- Bu, T.; Zhao, S.; Bai, F.; Sun, X.; He, K.; Wang, Q.; Jia, P.; Tian, Y.; Zhang, M.; Wang, L. Diverse Dyes-Embedded Staphylococcus aureus as Potential Biocarriers for Enhancing Sensitivity in Biosensing. Anal. Chem. 2021, 93, 6731–6738. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, X.; Liu, L.; Xu, L.; Kuang, H.; Xu, C.; Kang, H. An immunochromatographic strip sensor for sildenafil and its analogues. J. Mater. Chem. B 2019, 7, 6383–6389. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, S.; Wu, X.; Liu, L.; Kuang, H. A colloidal gold immunochromatography test strip based on a monoclonal antibody for the rapid detection of triadimefon and triadimenol in foods. Food Agric. Immunol. 2020, 31, 447–462. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Zeng, L.; Yang, H.; Zhong, Y.; Wang, J.; Ling, S.; Saeed, A.F.U.H.; Yuan, J.; Wang, S. Detection of okadaic acid (OA) using ELISA and colloidal gold immunoassay based on monoclonal antibody. J. Hazard. Mater. 2017, 339, 154–160. [Google Scholar] [CrossRef]
- Fu, J.M.; Zhou, Y.F.; Huang, X.L.; Zhang, W.J.; Wu, Y.H.; Fang, H.; Zhang, C.Z.; Xiong, Y.H. Dramatically Enhanced lmmunochromatographic Assay Using Cascade Signal Amplification for Ultrasensitive Detection of Escherichia coil O157:K7 in Milk. J. Agric. Food Chem. 2020, 68, 1118–1125. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Rhim, W.-K.; Kim, G.-H.; Nam, J.-M. Oxidative Nanopeeling Chemistry-Based Synthesis and Photodynamic and Photothermal Therapeutic Applications of Plasmonic Core-Petal Nanostructures. J. Am. Chem. Soc. 2014, 136, 16317–16325. [Google Scholar] [CrossRef]
- Zhang, G.-G.; Lai, X.-C.; Ding, N.-S.; Xiong, Q.-R.; Hou, S.; Duan, H.-W.; Lai, W.-H. Chrysanthemum-like Au@Polydopamine synthesized using one-pot method and its advantage in immunochromatographic assay. Sens. Actuators B Chem. 2021, 343, 130097. [Google Scholar] [CrossRef]
- Xie, Q.-Y.; Wu, Y.-H.; Xiong, Q.; Xu, H.-Y.; Xiong, Y.-H.; Liu, K.; Jin, Y.; Lai, W. Advantages of fluorescent microspheres compared with colloidal gold as a label in immunochromatographic lateral flow assays. Biosens. Bioelectron. 2014, 54, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xu, X.; Guo, L.; Wang, Z.; Ding, H.; Song, S.; Xu, L.; Kuang, H.; Liu, L.; Xu, C. An immunochromatographic sensor for ultrasensitive and direct detection of histamine in fish. J. Hazard. Mater. 2021, 419, 126533. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Hu, S.; Wang, C.; Yuan, M.; Huang, Z.; Xing, K.; Liu, D.; Peng, J.; Lai, W. Lateral flow immunoassays combining enrichment and colorimetry-fluorescence quantitative detection of sulfamethazine in milk based on trifunctional magnetic nanobeads. Food Control 2018, 98, 268–273. [Google Scholar] [CrossRef]
- Morais, E.C.; Collins, C.H.; Cristina, S.F.J.I. Pesticide determination in sweet peppers using QuEChERS and LC–MS/MS. Food Chem. 2018, 249, 77–83. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, Y.; Qu, X.; Zhou, L.; Yue, T.; Yuan, Y. Preparation of species-specific monoclonal antibody and development of fluorescence immunoassay based on fluorescence resonance energy transfer of carbon dots for accurate and sensitive detection of Alicyclobacillus acidoterrestris in apple juice. Food Chem. 2021, 347, 129069. [Google Scholar] [CrossRef]
- Carneiro, R.P.; Oliveira, F.A.; Madureira, F.D.; Silva, G.; de Souza, W.R.; Lopes, R. Development and method validation for determination of 128 pesticides in bananas by modified QuEChERS and UHPLC–MS/MS analysis. Food Control 2013, 33, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Chamkasem, N.; Ollis, L.W.; Harmon, T.; Lee, S.; Mercer, G. Analysis of 136 Pesticides in Avocado Using a Modified QuEChERS Method with LC-MS/MS and GC-MS/MS. J. Agric. Food Chem. 2013, 61, 2315–2329. [Google Scholar] [CrossRef]
- Song, X.; Shukla, S.; Kim, M. An immunoliposome-based immunochromatographic strip assay for the rapid detection of Cronobacter species. J. Microbiol. Methods 2019, 159, 91–98. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Xu, C.; Kuang, H.; Sun, L. Integration of antibody-antigen and receptor-ligand reactions to establish a gold strip biosensor for detection of 33 β-lactam antibiotics. Sci. China Mater. 2021, 64, 2056–2066. [Google Scholar] [CrossRef]
- Cao, Z.; Zhao, H.; Cui, Y.; Zhang, L.; Tan, G.; Wang, B.; Li, Q.X. Development of a Sensitive Monoclonal Antibody-Based Enzyme-Linked Immunosorbent Assay for the Analysis of Paclobutrazol Residue in Wheat Kernel. J. Agric. Food Chem. 2014, 62, 1826–1831. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, X.; Ren, K.; Zhu, G.; Zhang, Z.; Wang, J.; Du, D. Highly efficient detection of paclobutrazol in environmental water and soil samples by time-resolved fluoroimmunoassay. Sci. Total Environ. 2016, 569–570, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, L.; Memon, A.; Zhou, X.; Zhao, H. Waveguide-Based Fluorescent Immunosensor for the Simultaneous Detection of Carbofuran and 3-Hydroxy-Carbofuran. Biosensors 2020, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Wang, M.; Ding, S.; Fan, Y.; Diao, X.; Li, Q.X.; Zhao, H. Simultaneous detection of carbofuran and 3-hydroxy-carbofuran in vegetables and fruits by broad-specific monoclonal antibody-based ELISA. Food Agric. Immunol. 2019, 30, 1085–1096. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, Q.; Chen, S.; Liu, X.; Li, P. A double-label time-resolved fluorescent strip for rapidly quantitative detection of carbofuran residues in agro-products. Food Chem. 2017, 231, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liu, L.; Song, S.; Kuang, H.; Xu, C. Development of indirect competitive enzyme-linked immunosorbent and immunochromatographic strip assays for carbofuran detection in fruits and vegetables. Food Agric. Immunol. 2017, 28, 639–651. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.; Sun, W.; Chen, L.; Zhou, H.; Fan, Y.; Diao, X.; Wang, B.; Zhao, H. Simultaneous and rapid detection of carbofuran and 3-hydroxy-carbofuran in water samples and pesticide preparations using lateral-flow immunochromatographic assay. Food Agric. Immunol. 2020, 31, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Narenderan, S.; Meyyanathan, S.; Babu, B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction and detection techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Maštovská, K.; Lightfield, A.R. Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables. J. Aoac Int. 2005, 88, 615–629. [Google Scholar] [CrossRef] [Green Version]






| Methods | Sample | Analyte | LOD | Assay Time | Reference |
|---|---|---|---|---|---|
| Enzyme immunoassay | Wheat kernel | PBZ | 2.0 ng g−1 | >1 h | [40] |
| Time-resolved fluoroimmunoassay | Water and soil | PBZ | 1.09 ng mL−1 | >1 h | [41] |
| This work | Orange, grape, and cabbage mustard | PBZ | 0.117, 0.109, 0.131 μg kg−1 | 15 min | |
| Fluorescent immunosensor | Water and agricultural products | CAR | 0.13 μg L−1 | 30 min | [42] |
| ELISA | Vegetables and fruits | CAR | 0.76 ng mL−1 | >1 h | [43] |
| Time-resolved fluorescent immunochromatographic assay | Green bean, cabbage, apple, and pear | CAR | 0.04–0.76 mg L−1 | 30 min | [44] |
| ELISA | Cucumbers and apples | CAR | 1 ng mL−1 | >1 h | [45] |
| Lateral-flow immunochromatographic assay | CAR | 7 ng mL−1 | 15 min | [46] | |
| This work | Orange, grape and cabbage mustard | CAR | 0.087, 0.056, 0.094 μg kg−1 | 15 min |
| Sample | Spiked (μg kg−1) | Measured (μg kg−1) | Recovery (μg kg−1) | CV (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Oranges | PBZ | CAR | PBZ | CAR | PBZ | CAR | PBZ | CAR |
| 0.5 | 0.5 | 0.4978 | 0.5121 | 99.56 | 102.42 | 10.12 | 9.71 | |
| 5 | 5 | 5.1415 | 4.8934 | 102.83 | 97.86 | 8.94 | 9.69 | |
| 20 | 10 | 19.8311 | 10.0683 | 99.15 | 100.68 | 11.05 | 10.31 | |
| Grapes | PBZ | CAR | PBZ | CAR | PBZ | CAR | PBZ | CAR |
| 0.5 | 0.5 | 0.5009 | 0.5082 | 100.18 | 101.60 | 9.74 | 8.97 | |
| 5 | 5 | 5.0171 | 5.0632 | 100.34 | 101.26 | 8.96 | 9.73 | |
| 20 | 10 | 20.0854 | 9.8681 | 100.42 | 98.68 | 10.82 | 10.05 | |
| Cabbage mustard | PBZ | CAR | PBZ | CAR | PBZ | CAR | PBZ | CAR |
| 0.5 | 0.5 | 0.5053 | 0.5069 | 101.06 | 101.38 | 10.43 | 9.94 | |
| 5 | 5 | 4.9821 | 5.0736 | 99.64 | 101.47 | 9.61 | 10.08 | |
| 20 | 10 | 19.7931 | 10.0591 | 98.96 | 100.51 | 10.01 | 10.16 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Yan, Y.; Zhang, K.; Fu, H.; Lu, M.; Zhu, H.; Wei, D.; Peng, J.; Lai, W. Novel Dual-Color Immunochromatographic Assay Based on Chrysanthemum-like Au@polydopamine and Colloidal Gold for Simultaneous Sensitive Detection of Paclobutrazol and Carbofuran in Fruits and Vegetables. Foods 2022, 11, 1564. https://doi.org/10.3390/foods11111564
Yin J, Yan Y, Zhang K, Fu H, Lu M, Zhu H, Wei D, Peng J, Lai W. Novel Dual-Color Immunochromatographic Assay Based on Chrysanthemum-like Au@polydopamine and Colloidal Gold for Simultaneous Sensitive Detection of Paclobutrazol and Carbofuran in Fruits and Vegetables. Foods. 2022; 11(11):1564. https://doi.org/10.3390/foods11111564
Chicago/Turabian StyleYin, Jiaqi, Yiyong Yan, Kezhuo Zhang, Hui Fu, Min Lu, Hai Zhu, Daixian Wei, Juan Peng, and Weihua Lai. 2022. "Novel Dual-Color Immunochromatographic Assay Based on Chrysanthemum-like Au@polydopamine and Colloidal Gold for Simultaneous Sensitive Detection of Paclobutrazol and Carbofuran in Fruits and Vegetables" Foods 11, no. 11: 1564. https://doi.org/10.3390/foods11111564