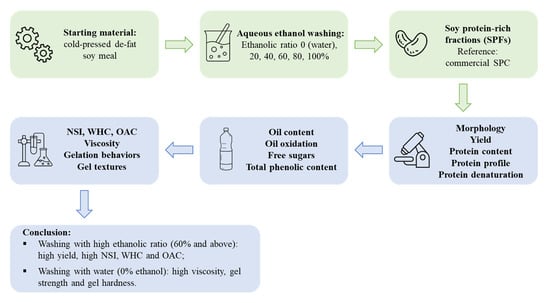
Characteristics of Soy Protein Prepared Using an Aqueous Ethanol Washing Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Aqueous Ethanol Washing Process
2.3. Microscopic Analysis
2.4. Composition and Yields
2.5. Protein Molecule Profile with SDS-PAGE
2.6. Oil Oxidation: Primary and Secondary Products
2.6.1. Hydroperoxide Concentration
2.6.2. Para-Anisidine Value (pAV)
2.7. Total Phenolic Content (TPC) in the Extract
2.8. Particle Size Distribution
2.9. Differential Scanning Calorimetry (DSC)
2.10. Nitrogen Solubility Index (NSI)
2.11. Water Holding Capacity (WHC) and Oil Absorption Capacity (OAC)
2.12. Viscosity Analysis
2.13. Gelation Behaviors
2.14. Textural Analysis of Soy Protein Gels
2.15. Statistical Analysis
3. Results
3.1. Protein Content, Yield, Composition, and Microstructure
3.2. Oil Content and Oil Oxidation
3.3. Total Free Sugars
3.4. Protein Denaturation in SPCs
3.5. Particle Size Distribution (PSD)
3.6. Nitrogen Solubility Index (NSI)
3.7. Water Holding and Oil Absorption Capacity
3.8. Viscosity Analysis
3.9. Gelation Behaviors
3.10. Textural Analysis of Soy Gels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, K. Soybean as a powerhouse of nutrients and phytochemicals. In Soybeans as Functional Foods and Ingredients; Liu, K., Ed.; AOCS Press: Champaign, IL, USA, 2004; pp. 1–22. [Google Scholar]
- Hoffman, J.R.; Falvo, M.J. Protein—Which is best? J. Sport. Sci. Med. 2004, 3, 118–130. [Google Scholar]
- FAO. General Standard for Soy Protein Products. Codex Stand; FAO: Rome, Italy, 1989. [Google Scholar]
- Kinsella, J.E.; Damodaran, S.; German, B. Seed storage proteins. In New Protein Foods; Altschul Aaron, M., Wilcke, H.L., Eds.; Academic Press: Cambridge, MA, USA, 1985; pp. 107–179. ISBN 978-0-12-054805-7. [Google Scholar]
- Preece, K.E.E.; Hooshyar, N.; Zuidam, N.J.J. Whole soybean protein extraction processes: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Chajuss, D. Soy protein concentrate: Technology, properties, and applications. In Soybeans as Functional Foods and Ingredients; Liu, K., Ed.; AOCS Press: Champaign, IL, USA, 2004; pp. 121–133. [Google Scholar]
- Chajuss, D. Soy protein concentrate: Processing, properties and prospects. Inf.-Int. News Fats Oils Relat. Mater. 2001, 12, 1176–1180. [Google Scholar]
- Hancock, J.D.; Peo, E.R.; Lewis, A.J.; Moxley, R.A. Effects of ethanol extraction and heat treatment of soybean flakes on function and morphology of pig intestine. J. Anim. Sci. 1990, 68, 3244–3251. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.A.; Lusas, E.W. Comparison of alternative solvents for oils extraction. J. Am. Oil Chem. Soc. 1983, 60, 229–242. [Google Scholar] [CrossRef]
- L’hocine, L.; Boye, J.I.; Arcand, Y. Composition and functional properties of soy protein isolates prepared using alternative defatting and extraction procedures. J. Food Sci. 2006, 71, C137–C145. [Google Scholar] [CrossRef]
- Koubaa, M.; Mhemdi, H.; Vorobiev, E. Influence of canola seed dehulling on the oil recovery by cold pressing and supercritical CO2 extraction. J. Food Eng. 2016, 182, 18–25. [Google Scholar] [CrossRef]
- Fetzer, A.; Herfellner, T.; Stäbler, A.; Menner, M.; Eisner, P. Influence of process conditions during aqueous protein extraction upon yield from pre-pressed and cold-pressed rapeseed press cake. Ind. Crops Prod. 2018, 112, 236–246. [Google Scholar] [CrossRef]
- Krul, E.S. Calculation of nitrogen-to-protein conversion factors: A review with a focus on soy protein. J. Am. Oil Chem. Soc. 2019, 96, 339–364. [Google Scholar] [CrossRef]
- McCleary, B.V.; Charnock, S.J.; Rossiter, P.C.; O’Shea, M.F.; Power, A.M.; Lloyd, R.M. Measurement of carbohydrates in grain, feed and food. J. Sci. Food Agric. 2006, 86, 1648–1661. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, Sensitive, Iron-Based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]
- Feng, J.; Schroën, K.; Fogliano, V.; Berton-Carabin, C. Antioxidant potential of non-modified and glycated soy proteins in the continuous phase of oil-in-water emulsions. Food Hydrocoll. 2021, 114, 106564. [Google Scholar] [CrossRef]
- AOCS Official Method Cd 18-90: P-Anisidine Value. Official Methods and Recommended Practices of the AOCS; AOCS: Boulder Urbana, IL, USA, 2017.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 152–178. [Google Scholar]
- Wang, C.; Johnson, L.A. Functional properties of hydrothermally cooked soy protein products. J. Am. Oil Chem. Soc. 2001, 78, 189–195. [Google Scholar] [CrossRef]
- Peters, J.P.C.M.; Vergeldt, F.J.; Boom, R.M.; van der Goot, A.J. Water-binding capacity of protein-rich particles and their pellets. Food Hydrocoll. 2017, 65, 144–156. [Google Scholar] [CrossRef]
- Lin, M.J.Y.; Humbert, E.S.; Sosullski, F.W. Certain functional properties of sunflower meal products. J. Food Sci. 1974, 39, 368–370. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N.; Olanya, O.M.; Uknalis, J.; Zhang, X.; Koplitz, B.D.; He, J. Surface characterization of cottonseed meal products by SEM, SEM-EDS, XRD and XPS Analysis. J. Mater. Sci. Res. 2017, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Kyriakopoulou, K.; Roelofs, B.; Ndiaye, M.; Vincken, J.-P.; Keppler, J.K.; van der Goot, A.J. Removal of phenolic compounds from de-oiled sunflower kernels by aqueous ethanol washing. Food Chem. 2021, 362, 130204. [Google Scholar] [CrossRef]
- Boulet, M.; Britten, M.; Lamarche, F. Dispersion of food proteins in water-alcohol mixed dispersants. Food Chem. 2001, 74, 69–74. [Google Scholar] [CrossRef]
- Iwabuchi, S.; Yamauchi, F. Electrophoretic analysis of whey proteins present in soybean globulin fractions. J. Agric. Food Chem. 1987, 35, 205–209. [Google Scholar] [CrossRef]
- Badley, R.A.; Atkinson, D.; Hauser, H.; Oldani, D.; Green, J.P.; Stubbs, J.M. The structure, physical and chemical properties of the soy bean protein glycinin. BBA. Protein Struct. 1975, 412, 214–228. [Google Scholar] [CrossRef]
- Liu, H.H.; Kuo, M.I. Ultra high pressure homogenization effect on the proteins in soy flour. Food Hydrocoll. 2016, 52, 741–748. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Yu, C.J.; Li, W.T.; Hsieh, J.F. Coagulation of β-conglycinin, glycinin and isoflavones induced by calcium chloride in soymilk. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, J.F.; Yu, C.J.; Tsai, T.Y. Proteomic profiling of the coagulation of soymilk proteins induced by magnesium chloride. Food Hydrocoll. 2012, 29, 219–225. [Google Scholar] [CrossRef]
- Duque-Estrada, P.; School, E.; van der Goot, A.J.; Berton-Carabin, C.C. Double emulsions for iron encapsulation: Is a high concentration of lipophilic emulsifier ideal for physical and chemical stability? J. Sci. Food Agric. 2019, 99, 4540–4549. [Google Scholar] [CrossRef] [Green Version]
- Nieh, C.D.; Snyder, H.E. Solvent extraction of oil from soybean flour II- pilot plant and two-solvent extractions. J. Am. Oil Chem. Soc. 1991, 68, 250–253. [Google Scholar] [CrossRef]
- Gandhi, A.P.; Joshi, K.C.; Jha, K.; Parihar, V.S.; Srivastav, D.C.; Raghunadh, P.; Kawalkar, J.; Jain, S.K.; Tripathi, R.N. Studies on alternative solvents for the extraction of oil-l soybean. Int. J. Food Sci. Technol. 2003, 38, 369–375. [Google Scholar] [CrossRef]
- Schaich, K.M.; Shahidi, F.; Zhong, Y.; Eskin, N.A.M. Lipid oxidation. In Biochemistry of Foods; Elsevier: Amsterdam, The Netherlands, 2012; pp. 419–478. ISBN 9780080918099. [Google Scholar]
- Meissner, P.M.; Keppler, J.K.; Stöckmann, H.; Schwarz, K. Cooxidation of proteins and lipids in whey protein oleogels with different water amounts. Food Chem. 2020, 328, 127123. [Google Scholar] [CrossRef] [PubMed]
- De Moura Bell, J.M.L.N.; Maurer, D.; Yao, L.; Wang, T.; Jung, S.; Johnson, L.A. Characteristics of oil and skim in enzyme-assisted aqueous extraction of soybeans. J. Am. Oil Chem. Soc. 2013, 90, 1079–1088. [Google Scholar] [CrossRef]
- Duque-Estrada, P.; Kyriakopoulou, K.; de Groot, W.; van der Goot, A.J.; Berton-Carabin, C.C. Oxidative stability of soy proteins: From ground soybeans to structured products. Food Chem. 2020, 318, 126499. [Google Scholar] [CrossRef]
- Bellaloui, N. Soybean seed phenol, lignin, and isoflavones and sugars composition altered by foliar boron application in soybean under water stress. Food Nutr. Sci. 2012, 03, 579–590. [Google Scholar] [CrossRef] [Green Version]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: A detailed review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Wolf, W.J. Lipoxygenase and flavor of soybean protein products. J. Agric. Food Chem. 1975, 23, 136–141. [Google Scholar] [CrossRef]
- Borhan, M.; Snyder, H.E. Lipoxygenase destruction in whole soybeans by combinations of heating and soaking in ethanol. J. Food Sci. 1979, 44, 586–590. [Google Scholar] [CrossRef]
- Johansen, H.N.; Glitsø, V.; Bach Knudsen, K.E. Influence of extraction solvent and temperature on the quantitative determination of oligosaccharides from plant materials by high-performance liquid chromatography. J. Agric. Food Chem. 1996, 44, 1470–1474. [Google Scholar] [CrossRef]
- Choct, M.; Dersjant-Li, Y.; McLeish, J.; Peisker, M. Soy oligosaccharides and soluble non-starch polysaccharides: A review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian-Australas. J. Anim. Sci. 2010, 23, 1386–1398. [Google Scholar] [CrossRef]
- Ekvall, J.; Stegmark, R.; Nyman, M. Optimization of extraction methods for determination of the raffinose family oligosaccharides in leguminous vine peas (Pisum sativum L.) and effects of blanching. J. Food Compos. Anal. 2007, 20, 13–18. [Google Scholar] [CrossRef]
- Hashizume, K.; Watanabe, T. Influence of heating temperature on conformational changes of soybean proteinst. Agric. Biol. Chem. 1979, 43, 683–690. [Google Scholar] [CrossRef]
- Griebenow, K.; Klibanov, A.M. On protein denaturation in aqueous-organic mixtures but not in pure organic solvents. J. Am. Chem. Soc. 1996, 118, 11695–11700. [Google Scholar] [CrossRef]
- Roberts, R.C.; Briggs, D.R. Characteristics of the various soybean globulin components with respect to denaturation by ethanol. Cereal Chem. 1963, 40, 450–458. [Google Scholar]
- Ishino, K.; Kudo, S. Conformational transition of alkali-denatured soybean 7S and 11S globulins by ethanol. Agric. Biol. Chem. 1980, 44, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Devi, V.S.; Chidi, O.O.; Coleman, D. Dominant effect of ethanol in thermal destabilization of bovine serum albumin in the presence of sucrose. Spectroscopy 2009, 23, 265–270. [Google Scholar] [CrossRef]
- Kulmyrzaev, A.; Bryant, C.; McClements, D.J. Influence of sucrose on the thermal denaturation, gelation, and emulsion stabilization of whey proteins. J. Agric. Food Chem. 2000, 48, 1593–1597. [Google Scholar] [CrossRef]
- Peng, L.P.; Xu, Y.T.; Li, X.T.; Tang, C.H. Improving the emulsification of soy β-conglycinin by alcohol-induced aggregation. Food Hydrocoll. 2020, 98, 105307. [Google Scholar] [CrossRef]
- Kant Yadav, J.; Chandani, N.; Prajakt PR, P.; Bala Chauhan, J. Counter Effect of sucrose on ethanol-induced aggregation of protein. Protein Pept. Lett. 2012, 17, 1542–1546. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.M.; Lozano, J.M.; Sancho, J.; Giraldo, G.I. Thermal stability of β-lactoglobulin in the presence of aqueous solution of alcohols and polyols. Int. J. Biol. Macromol. 2007, 40, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Narizhneva, N.V.; Kirschstein, S.O.; Winter, S.; Löber, G. Conformational transitions provoked by organic solvents in β-lactoglobulin: Can a molten globule like intermediate be induced by the decrease in dielectric constant? Fold. Des. 1997, 2, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.M.; Cabello-Villegas, J.; Hutchings, R.L.; Mallela, K.M.G. Role of partial protein unfolding in alcohol-induced protein aggregation. Proteins Struct. Funct. Bioinform. 2010, 78, 2625–2637. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Stone, A.K.; Green, R.; Nickerson, M.T. Reduction of off-flavours and the impact on the functionalities of lentil protein isolate by acetone, ethanol, and isopropanol treatments. Food Chem. 2019, 277, 84–95. [Google Scholar] [CrossRef]
- Sawada, M.M.; Venâncio, L.L.; Toda, T.A.; Rodrigues, C.E.C. Effects of different alcoholic extraction conditions on soybean oil yield, fatty acid composition and protein solubility of defatted meal. Food Res. Int. 2014, 62, 662–670. [Google Scholar] [CrossRef]
- Cornet, S.H.V.; Snel, S.J.E.; Lesschen, J.; van der Goot, A.J.; van der Sman, R.G.M. Enhancing the water holding capacity of model meat analogues through marinade composition. J. Food Eng. 2021, 290, 110283. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Melachouris, N. Functional properties of proteins in foods: A survey. Crit. Rev. Food Sci. Nutr. 1976, 7, 219–280. [Google Scholar] [CrossRef]
- Peng, Y.; Kyriakopoulou, K.; Rahmani, A.; Venema, P.; van der Goot, A.J. Isochoric moisture heating as a tool to control the functionality of soy protein. LWT 2021, 111979. [Google Scholar] [CrossRef]
- Wang, Y.; Guldiken, B.; Tulbek, M.; House, J.D.; Nickerson, M. Impact of alcohol washing on the flavour profiles, functionality and protein quality of air classified pea protein enriched flour. Food Res. Int. 2020, 132, 109085. [Google Scholar] [CrossRef]
- Kornet, C.; Venema, P.; Nijsse, J.; van der Linden, E.; van der Goot, A.J.; Meinders, M. Yellow pea aqueous fractionation increases the specific volume fraction and viscosity of its dispersions. Food Hydrocoll. 2020, 99, 105332. [Google Scholar] [CrossRef]
- Nicole, M.; Caimeng, Z.; Joseph, H.; Eric, K.; Yufei, H. Soybean oil volume fraction effects on the rheology characteristics and gelation behavior of glucono-δ-Lactone and calcium sulfate-induced tofu gels. J. Texture Stud. 2016, 47, 112–130. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, B.; Hemar, Y.; Chen, J.; Cui, B. Improvement of calcium sulfate-induced gelation of soy protein via incorporation of soy oil before and after thermal denaturation. LWT 2020, 117, 108690. [Google Scholar] [CrossRef]
- Sok, L.T.; Kasapis, S.; Perera, C.O.; Barlow, P.J. Functional and structural properties of 2S soy protein in relation to other molecular protein fractions. J. Agric. Food Chem. 2006, 54, 6046–6053. [Google Scholar] [CrossRef]
- Ould Eleya, M.M.; Ko, S.; Gunasekaran, S. Scaling and fractal analysis of viscoelastic properties of heat-induced protein gels. Food Hydrocoll. 2004, 18, 315–323. [Google Scholar] [CrossRef]
- Hesarinejad, M.A.; Koocheki, A.; Razavi, S.M.A. Dynamic rheological properties of lepidium perfoliatum seed gum: Effect of concentration, temperature and heating/cooling rate. Food Hydrocoll. 2014, 35, 583–589. [Google Scholar] [CrossRef]
- Yamano, Y.; Miki, E.; Fukui, Y. Effect of palm oil on the texture of soybean protein gel. Nippon. Shokuhin Kogyo Gakkaishi 1981, 28, 131–135. [Google Scholar] [CrossRef]
- Kornet, R.; Veenemans, J.; Venema, P.; van der Goot, A.J.; Meinders, M.; Sagis, L.; van der Linden, E. Less is more: Limited fractionation yields stronger gels for pea proteins. Food Hydrocoll. 2021, 112, 106285. [Google Scholar] [CrossRef]












| Water/Ethanol Ratios | Protein Content of SPC, db.% | Solid Content of the SPC Samples, % | ||
|---|---|---|---|---|
| 6 wt% Protein Concentration | 9 wt% Protein Concentration | 15 wt% Protein Concentration | ||
| 0% | 46.48 ± 0.29 | 12.91 ± 0.08 | 19.36 ± 0.12 | 32.27 ± 0.20 |
| 20% | 56.43 ± 0.47 | 10.63 ± 0.09 | 15.95 ± 0.13 | 26.58 ± 0.22 |
| 40% | 59.49 ± 1.11 | 10.09 ± 0.19 | 15.13 ± 0.28 | 25.22 ± 0.47 |
| 60% | 57.36 ± 0.60 | 10.46 ± 0.11 | 15.69 ± 0.16 | 26.15 ± 0.27 |
| 80% | 52.10 ± 0.92 | 11.52 ± 0.20 | 17.27 ± 0.31 | 28.79 ± 0.51 |
| 100% | 50.70 ± 0.21 | 11.83 ± 0.05 | 17.75 ± 0.07 | 29.58 ± 0.12 |
| Commercial SPC | 61.68 ± 0.00 | 9.73 ± 0.00 | 14.59 ± 0.00 | 24.32 ± 0.00 |
| Water/Ethanol Ratios | 7S Td (°C) | Enthalpy (J/g) | 11S Td (°C) | Enthalpy (J/g) |
|---|---|---|---|---|
| 0% | 75.72 ± 0.44 a | 1.73 ± 0.36 a | 94.30 ± 0.21 d | 3.31 ± 0.31 ab |
| 20% | 75.20 ± 0.19 a | 1.85 ± 0.12 a | 95.05 ± 0.04 c | 3.59 ± 0.64 ab |
| 40% | 75.94 ± 1.45 a | 0.70 ± 0.04 c | 92.44 ± 0.19 e | 3.98 ± 0.74 a |
| 60% | 74.64 ± 1.56 a | 0.75 ± 0.13 c | 92.74 ± 0.49 e | 3.38 ± 0.27 ab |
| 80% | 76.11 ± 0.33 a | 1.33 ± 0.07 b | 95.76 ± 0.09 b | 3.48 ± 0.14 ab |
| 100% | 76.08 ± 0.14 a | 1.19 ± 0.14 b | 96.73 ± 0.27 a | 3.14 ± 0.31 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Kyriakopoulou, K.; Ndiaye, M.; Bianeis, M.; Keppler, J.K.; van der Goot, A.J. Characteristics of Soy Protein Prepared Using an Aqueous Ethanol Washing Process. Foods 2021, 10, 2222. https://doi.org/10.3390/foods10092222
Peng Y, Kyriakopoulou K, Ndiaye M, Bianeis M, Keppler JK, van der Goot AJ. Characteristics of Soy Protein Prepared Using an Aqueous Ethanol Washing Process. Foods. 2021; 10(9):2222. https://doi.org/10.3390/foods10092222
Chicago/Turabian StylePeng, Yu, Konstantina Kyriakopoulou, Mbalo Ndiaye, Marine Bianeis, Julia K. Keppler, and Atze Jan van der Goot. 2021. "Characteristics of Soy Protein Prepared Using an Aqueous Ethanol Washing Process" Foods 10, no. 9: 2222. https://doi.org/10.3390/foods10092222