Fresh Mushroom Preservation Techniques
Abstract
:1. Introduction
2. Characteristics of Mushrooms
2.1. Chemical Composition and Nutritional Value
2.2. Nutraceutical and Therapeutic Properties
3. Factors Affecting the Quality of Fresh Mushrooms
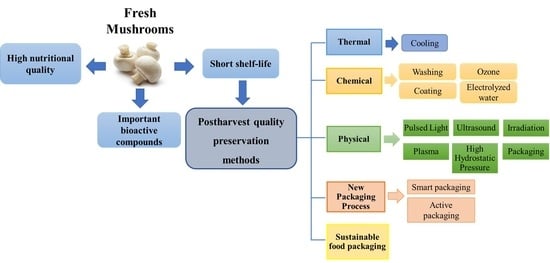
4. Conservation Methods for Fresh Mushrooms
4.1. Thermal Processes
Cooling
4.2. Chemical Treatments
4.2.1. Washing with Antimicrobial and Antibrowning Agents
4.2.2. Coatings
4.2.3. Ozone
4.2.4. Electrolyzed Water
4.3. Physical Treatments
4.3.1. Pulsed Light
4.3.2. Ultrasound
4.3.3. Irradiation
4.3.4. Plasma
4.3.5. High Hydrostatic Pressure (HHP)
4.3.6. Packaging
Modified-Atmosphere Packaging (MAP)
Microperforated Food Packaging and Moisture Regulators
5. New Packaging Processes
5.1. Active Packaging
5.2. Intelligent Packaging
6. Sustainable Food Packaging
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roncero-Ramos, I.; Delgado-Andrade, C. The beneficial role of edible mushrooms in human health. Curr. Opin. Food Sci. 2017, 14, 122–128. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of United Nations (FAO). FAOSTAT Database. Available online: http://www.fao.org/faostat/en/#data/QC/%0A (accessed on 8 August 2021).
- Siwulski, M.; Budka, A.; Rzymski, P.; Gąsecka, M.; Kalač, P.; Budzyńska, S.; Magdziak, Z.; Niedzielski, P.; Mleczek, P.; Mleczek, M. Worldwide basket survey of multielemental composition of white button mushroom Agaricus bisporus. Chemosphere 2020, 239, 124718. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C.F.R. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Royse, D.J. A Global Perspective on the High Five: Agaricus, Pleurotus. In Proceedings of the International Conference on Mushroom Biology and Mushroom Products, New Delhi, India, 19–22 November 2014; pp. 2010–2015. [Google Scholar]
- Diamantopoulou, P.; Philippoussis, A. Cultivated Mushrooms: Preservation and Processing. In Handbook of Vegetable Preservation and Processing; CRC Press: Boca Raton, FL, USA, 2015; pp. 495–517. [Google Scholar]
- Subramaniam, S.; Jiao, S.; Zhang, Z.; Jing, P. Impact of post-harvest processing or thermal dehydration on physiochemical, nutritional and sensory quality of shiitake mushrooms. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2560–2595. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, M.; Shan, L.; You, Y.; Salokhe, V.M. Extension of the shelf-life of fresh oyster mushrooms (Pleurotus ostreatus) by modified atmosphere packaging with chemical treatments. African J. Biotechnol. 2011, 10, 9509–9517. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T. Effect of alginate coating on physicochemical and sensory qualities of button mushrooms (Agaricus bisporus) under a high oxygen modified atmosphere. Postharvest Biol. Technol. 2013, 76, 91–97. [Google Scholar] [CrossRef]
- Antmann, G.; Lareo, C.; Ares, G.; Lema, P. Influence of Temperature on Respiration Rate of Shiitake Mushrooms under Air and 15 % O2. Fresh Prod. 2008, 2, 14–16. [Google Scholar]
- Pei, F.; Shi, Y.; Gao, X.; Wu, F.; Mariga, A.M.; Yang, W.; Zhao, L.; An, X.; Xin, Z.; Yang, F.; et al. Changes in non-volatile taste components of button mushroom (Agaricus bisporus) during different stages of freeze drying and freeze drying combined with microwave vacuum drying. Food Chem. 2014, 165, 547–554. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Ayeka, P.A. Potential of Mushroom Compounds as Immunomodulators in Cancer Immunotherapy: A Review. Evid.-based Complement. Altern. Med. 2018, 2018, 7271509. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Beulah, G.H.; Margret, A.A.; Nelson, J. Marvelous Medicinal Mushrooms. Int. J. Pharm. Biol. Sci. 2013, 3, 611–615. [Google Scholar]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.P.; Annepu, S.K.; Gautam, Y.; Singh, M.; Kamal, S. Status of mushroom production in India. Mushroom Res. 2017, 26, 111–120. [Google Scholar]
- Gautam, A.S. Recent Advancements in Sciences with Special Reference to Himalaya, 1st ed.; Gautam, A.S., Ed.; Ancient Publishing House: New Delhi, India, 2020; ISBN 9789384866914. [Google Scholar]
- Hrudayanath, T.; Sameer, K.S. Diversity, nutritional composition and medicinal potential of Indian mushrooms: A review. African J. Biotechnol. 2014, 13, 523–545. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 1–14, 376387. [Google Scholar] [CrossRef]
- Roncero Ramos, I. Nutritional and Health Properties of Mushrooms; Mushroom Research Technological Center of La Rioja (CETICH): La Rioja, Spain, 2015; pp. 12–23. [Google Scholar]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Wani, B.A.; Bodha, R.H.; Wani, A.H. Nutritional and medicinal importance of mushrooms. J. Med. Plants Res. 2010, 4, 2598–2604. [Google Scholar] [CrossRef] [Green Version]
- Roupas, P.; Keogh, J.; Noakes, M.; Margetts, C.; Taylor, P. The role of edible mushrooms in health: Evaluation of the evidence. J. Funct. Foods 2012, 4, 687–709. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci. Hum. Wellness 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nishimura, M.; Sato, Y.; Sato, H.; Nishihira, J. Enhancement of the Th1-phenotype immune system by the intake of Oyster mushroom (Tamogitake) extract in a double-blind, placebo-controlled study. J. Tradit. Complement. Med. 2016, 6, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Cheng, M.; Dong, L.; Yang, K.; Ma, Z.; Yu, S.; Yan, P.; Bai, K.; Zhu, X.; Zhang, Q. Agaricus blazei extract (FA-2-b-β) induces apoptosis in chronic myeloid leukemia cells. Oncol. Lett. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D’Arrigo, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A review of mushrooms as a potential source of dietary vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [Green Version]
- Taofiq, O.; Fernandes, Â.; Barros, L.; Barreiro, M.F.; Ferreira, I.C.F.R. UV-irradiated mushrooms as a source of vitamin D2: A review. Trends Food Sci. Technol. 2017, 70, 82–94. [Google Scholar] [CrossRef]
- Black, L.J.; Lucas, R.M.; Sherriff, J.L.; Björn, L.O.; Bornman, J.F. In pursuit of vitamin D in plants. Nutrients 2017, 9, 136. [Google Scholar] [CrossRef] [Green Version]
- Mohamed Yahaya, N.F.; Rahman, M.A.; Abdullah, N. Therapeutic potential of mushrooms in preventing and ameliorating hypertension. Trends Food Sci. Technol. 2014, 39, 104–115. [Google Scholar] [CrossRef]
- Phan, C.W.; David, P.; Sabaratnam, V. Edible and Medicinal Mushrooms: Emerging Brain Food for the Mitigation of Neurodegenerative Diseases. J. Med. Food 2017, 20, 1–10. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, S.; Sun, Y.; Zhang, Q. Medicinal properties of Hericium erinaceus and its potential to formulate novel mushroom-based pharmaceuticals. Appl. Microbiol. Biotechnol. 2014, 98, 7661–7670. [Google Scholar] [CrossRef]
- Rahman, M.A.; Abdullah, N.; Aminudin, N. Interpretation of mushroom as a common therapeutic agent for Alzheimer’s disease and cardiovascular diseases. Crit. Rev. Biotechnol. 2016, 36, 1131–1142. [Google Scholar] [CrossRef]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef]
- Taofiq, O.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Anti-inflammatory potential of mushroom extracts and isolated metabolites. Trends Food Sci. Technol. 2016, 50, 193–210. [Google Scholar] [CrossRef] [Green Version]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Li, Y.; Zhou, T.; Xu, D.P.; Zhang, P.; Li, S.; Li, H.-B. Bioactivities and health benefits of mushrooms mainly from China. Molecules 2016, 21, 938. [Google Scholar] [CrossRef] [Green Version]
- Robaszkiewicz, A.; Bartosz, G.; Ławrynowicz, M.; Soszyński, M. The role of polyphenols, β -carotene, and lycopene in the antioxidative action of the extracts of dried, edible mushrooms. J. Nutr. Metab. 2010, 173274. [Google Scholar] [CrossRef] [Green Version]
- Farokhian, F.; Jafarpour, M.; Goli, M.; Askari-Khorasgani, O. Quality Preservation of Air-Dried Sliced Button Mushroom (Agaricus bisporus) by Lavender (Lavendula angustifolia Mill.) Essential Oil. J. Food Process Eng. 2017, 40, e12432. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, M.; Singh, G. Effect of different pretreatments on the quality of mushrooms during solar drying. J. Food Sci. Technol. 2013, 50, 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Langowski, H.-C.; Wani, A.A.; Saengerlaub, S. Recent advances in extending the shelf life of fresh Agaricus mushrooms: A review. J. Sci. Food Agric. 2010, 90, 1393–1402. [Google Scholar] [CrossRef]
- Ares, G.; Parentelli, C.; Gámbaro, A.; Lareo, C.; Lema, P. Sensory shelf life of shiitake mushrooms stored under passive modified atmosphere. Postharvest Biol. Technol. 2006, 41, 191–197. [Google Scholar] [CrossRef]
- Aguirre, L.; Frias, J.M.; Barry-Ryan, C.; Grogan, H. Assessing the effect of product variability on the management of the quality of mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2008, 49, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, P.V.; Rodrigues, F.A.S.; Motel, A.; Leonhard, A. Development of a moisture absorber for packaging of fresh mushrooms (Agaricus bisporous). Postharvest Biol. Technol. 2008, 48, 408–414. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Oliveira, F.A.R.; Macedo, I. Effect of temperature and humidity on the transpiration rate of the whole mushrooms. J. Food Eng. 2008, 84, 281–288. [Google Scholar] [CrossRef]
- Gao, M.; Feng, L.; Jiang, T. Browning inhibition and quality preservation of button mushroom (Agaricus bisporus) by essential oils fumigation treatment. Food Chem. 2014, 149, 107–113. [Google Scholar] [CrossRef]
- Jiang, T.; Feng, L.; Wang, Y. Effect of alginate/nano-Ag coating on microbial and physicochemical characteristics of shiitake mushroom (Lentinus edodes) during cold storage. Food Chem. 2013, 141, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Han Lyn, F.; Maryam Adilah, Z.A.; Nor-Khaizura, M.A.R.R.; Jamilah, B.; Nur Hanani, Z.A. Application of modified atmosphere and active packaging for oyster mushroom (Pleurotus ostreatus). Food Packag. Shelf Life 2019, 23, 100451. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S.; Sharma, A. Free phenolics and polyphenol oxidase (PPO): The factors affecting post-cut browning in eggplant (Solanum melongena). Food Chem. 2013, 139, 105–114. [Google Scholar] [CrossRef]
- Quevedo, R.; Díaz, O.; Valencia, E.; Pedreschi, F.; Bastias, J.M.; Siche, R. Differences Between the Order Model and the Weibull Model in the Modeling of the Enzymatic Browning. Food Bioprocess Technol. 2016, 9, 1961–1967. [Google Scholar] [CrossRef]
- Wrona, M.; Bentayeb, K.; Nerín, C. A novel active packaging for extending the shelf-life of fresh mushrooms (Agaricus bisporus). Food Control 2015, 54, 200–207. [Google Scholar] [CrossRef]
- Jiang, T.; Feng, L.; Zheng, X. Effect of chitosan coating enriched with thyme oil on postharvest quality and shelf life of shiitake mushroom (Lentinus edodes). J. Agric. Food Chem. 2012, 60, 188–196. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, Y.; Zhang, J.; Liu, W.; Guan, W.; Wang, Z. Effects of high CO2 in-package treatment on flavor, quality and antioxidant activity of button mushroom (Agaricus bisporus) during postharvest storage. Postharvest Biol. Technol. 2017, 123, 112–118. [Google Scholar] [CrossRef]
- Zhang, K.; Pu, Y.Y.; Sun, D.W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Jaworska, G.; Pogoń, K.; Bernaś, E.; Skrzypczak, A. Effect of Different Drying Methods and 24-Month Storage on Water Activity, Rehydration Capacity, and Antioxidants in Boletus edulis Mushrooms. Dry. Technol. 2014, 32, 291–300. [Google Scholar] [CrossRef]
- Azevedo, S.; Cunha, L.M.; Fonseca, S.C. Modelling the influence of time and temperature on the respiration rate of fresh oyster mushrooms. Food Sci. Technol. Int. 2015, 21, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Lin, X.; Sun, D.W. Research advances in browning of button mushroom (Agaricus bisporus): Affecting factors and controlling methods. Trends Food Sci. Technol. 2019, 90, 63–75. [Google Scholar] [CrossRef]
- Rossouw, W.; Korsten, L. Cultivable microbiome of fresh white button mushrooms. Lett. Appl. Microbiol. 2017, 64, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schill, S.; Stessl, B.; Meier, N.; Tichy, A.; Wagner, M.; Ludewig, M. Microbiological safety and sensory quality of cultivated mushrooms (Pleurotus eryngii, pleurotus ostreatus and lentinula edodes) at retail level and post-retail storage. Foods 2021, 10, 816. [Google Scholar] [CrossRef]
- Wong, W.C.; Preece, T.F. Pseudomonas tolaasii in cultivated mushroom (Agaricus bisporus) crops: Effects of sodium hypochlorite on the bacterium and on blotch disease severity. J. Appl. Bacteriol. 1985, 58, 259–267. [Google Scholar] [CrossRef]
- Chun, S.C.; Ahn, Y.N.; Khan, S.M.; Chung, I.M.; Won, H.Y.; Jhune, C.S.; Park, Y.J. The microbial population in the air of cultivation facility of oyster mushrooms. J. Microbiol. 2012, 50, 1053–1057. [Google Scholar] [CrossRef]
- Whyte, P.; McGill, K.; Cowley, D.; Madden, R.H.; Moran, L.; Scates, P.; Carroll, C.; O’Leary, A.; Fanning, S.; Collins, J.D.; et al. Occurrence of Campylobacter in retail foods in Ireland. Int. J. Food Microbiol. 2004, 95, 111–118. [Google Scholar] [CrossRef]
- Johannessen, G.S.; Loncarevic, S.; Kruse, H. Bacteriological analysis of fresh produce in Norway. Int. J. Food Microbiol. 2002, 77, 199–204. [Google Scholar] [CrossRef]
- Rivera, C.S.; Blanco, D.; Oria, R.; Venturini, M.E. Diversity of culturable microorganisms and occurrence of Listeria monocytogenes and Salmonella spp. in Tuber aestivum and Tuber melanosporum ascocarps. Food Microbiol. 2010, 27, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.E.; Reyes, J.E.; Rivera, C.S.; Oria, R.; Blanco, D. Microbiological quality and safety of fresh cultivated and wild mushrooms commercialized in Spain. Food Microbiol. 2011, 28, 1492–1498. [Google Scholar] [CrossRef]
- Que, P.T.T.; Verlinden, B.; Nicolai, B. Effect of controlled atmosphere and storage temperature on the weight loss and cap colour of fresh mushrooms (Agaricus bisporus). Can Tho Univ. J. Sci. 2017, 06, 127–139. [Google Scholar] [CrossRef]
- Guillaume, C.; Schwab, I.; Gastaldi, E.; Gontard, N. Biobased packaging for improving preservation of fresh common mushrooms (Agaricus bisporus L.). Innov. Food Sci. Emerg. Technol. 2010, 11, 690–696. [Google Scholar] [CrossRef]
- Azevedo, S.; Cunha, L.M.; Oliveira, J.C.; Mahajan, P.V.; Fonseca, S.C. Modelling the influence of time, temperature and relative humidity conditions on the mass loss rate of fresh oyster mushrooms. J. Food Eng. 2017, 212, 108–112. [Google Scholar] [CrossRef]
- Rux, G.; Mahajan, P.V.; Geyer, M.; Linke, M.; Pant, A.; Saengerlaub, S.; Caleb, O.J. Application of humidity-regulating tray for packaging of mushrooms. Postharvest Biol. Technol. 2015, 108, 102–110. [Google Scholar] [CrossRef]
- Cliffe-Byrnes, V.; Cusack, A.; Mahajan, P.V.; O’Beirne, D. Effects of different humidity conditions on the quality of whole mushrooms (Agaricus bisporus)—implications for modified atmosphere packages. In Proceedings of the 37th Annual Food Science and Technology Research Conference, Cork, Ireland, 6–7 September 2007; Volume 136, pp. 23–42. [Google Scholar]
- Liu, Q.; Cui, X.; Song, Z.; Kong, W.; Kang, Y.; Kong, W.; Ng, T.B. Coating shiitake mushrooms (Lentinus edodes) with a polysaccharide from Oudemansiella radicata improves product quality and flavor during postharvest storage. Food Chem. 2021, 352, 129357. [Google Scholar] [CrossRef]
- Louis, E.; Villalobos-Carvajal, R.; Reyes-Parra, J.; Jara-Quijada, E.; Ruiz, C.; Andrades, P.; Gacitúa, J.; Beldarraín-Iznaga, T. Preservation of mushrooms (Agaricus bisporus) by an alginate-based-coating containing a cinnamaldehyde essential oil nanoemulsion. Food Packag. Shelf Life 2021, 28, 100662. [Google Scholar] [CrossRef]
- Verma, A.K.; Shivani, P.C.S.; Kumar, M.; Rani, N. Processing of mushrooms: A viable option to sustain the growing population of the developing countries. Int. J. Chem. Stud. 2020, 8, 1416–1423. [Google Scholar] [CrossRef]
- Aguirre, L.; Frias, J.M.; Barry-Ryan, C.; Grogan, H. Modelling browning and brown spotting of mushrooms (Agaricus bisporus) stored in controlled environmental conditions using image analysis. J. Food Eng. 2009, 91, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.; Nagasaka, R.; Ohshima, T. Effects of extraction solvents, cooking procedures and storage conditions on the contents of ergothioneine and phenolic compounds and antioxidative capacity of the cultivated mushroom Flammulina velutipes. Int. J. Food Sci. Technol. 2012, 47, 1193–1205. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Pu, H. Combining the genetic algorithm and successive projection algorithm for the selection of feature wavelengths to evaluate exudative characteristics in frozen-thawed fish muscle. Food Chem. 2016, 197, 855–863. [Google Scholar] [CrossRef]
- Brosnan, T.; Sun, D.W. Precooling techniques and applications for horticultural products—A review. Int. J. Refrig. 2001, 24, 154–170. [Google Scholar] [CrossRef]
- Ozturk, H.M.; Ozturk, H.K.; Koçar, G. Microbial analysis of meatballs cooled with vacuum and conventional cooling. J. Food Sci. Technol. 2017, 54, 2825–2832. [Google Scholar] [CrossRef]
- Kader, A.A.; Rolle, R.S. The role of post-harvest management in assuring the quality and safety of agricultural services. In FAO Agricultural Services Bulletin 152; FAO, Ed.; FAO, Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; ISBN 9251051372. [Google Scholar]
- Burton, K.S.; Frost, C.E.; Atkey, P.T. Effect of vacuum cooling on mushroom browning. Int. J. Food Sci. Technol. 1987, 22, 599–606. [Google Scholar] [CrossRef]
- Chand Mittal, T.; Sharma, S.R.; Jindal, N. Effect of Pre-Cooling and Packaging Materials Under Ambient Condition Storage on Postharvest Quality of White Button Mushroom. Ind. J. Sci. Res. Tech 2014, 2, 60–71. [Google Scholar]
- Tao, F.; Zhang, M.; Yu, H. qing Effect of vacuum cooling on physiological changes in the antioxidant system of mushroom under different storage conditions. J. Food Eng. 2007, 79, 1302–1309. [Google Scholar] [CrossRef]
- Brennan, M.; Le Port, G.; Gormley, R. Post-harvest Treatment with Citric Acid or Hydrogen Peroxide to Extend the Shelf Life of Fresh Sliced Mushrooms. LWT Food Sci. Technol. 2000, 33, 285–289. [Google Scholar] [CrossRef]
- Gupta, P.; Bhat, A. Efficacy of Different Washing Treatments on Quality of Button Mushrooms (A.bisporus). J. Food Process. Technol. 2016, 7, 590. [Google Scholar] [CrossRef]
- Guan, W.; Fan, X.; Yan, R. Effect of combination of ultraviolet light and hydrogen peroxide on inactivation of Escherichia coli O157: H7, native microbial loads, and quality of button mushrooms. Food Control 2013, 34, 554–559. [Google Scholar] [CrossRef]
- Sapers, G.M.; Miller, R.L.; Choi, S.W.; Cooke, P.H. Structure and composition of mushrooms as affected by hydrogen peroxide wash. J. Food Sci. 1999, 64, 889–892. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Xue, Z.; Hao, J.; Yu, W.; Kou, X. Effects of Processing and Storage Preservation Technologies on Nutritional Quality and Biological Activities of Edible Fungi: A Review. J. Food Process Eng. 2017, 40, e12437. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Application of Tragacanth gum impregnated with Satureja khuzistanica essential oil as a natural coating for enhancement of postharvest quality and shelf life of button mushroom (Agaricus bisporus). Int. J. Biol. Macromol. 2018, 106, 218–226. [Google Scholar] [CrossRef]
- Jiang, T.; Feng, L.; Zheng, X.; Li, J. Physicochemical responses and microbial characteristics of shiitake mushroom (Lentinus edodes) to gum arabic coating enriched with natamycin during storage. Food Chem. 2013, 138, 1992–1997. [Google Scholar] [CrossRef]
- Jiang, T.; Feng, L.; Li, J. Changes in microbial and postharvest quality of shiitake mushroom (Lentinus edodes) treated with chitosan-glucose complex coating under cold storage. Food Chem. 2012, 131, 780–786. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Tragacanth gum containing Zataria multiflora Boiss. essential oil as a natural preservative for storage of button mushrooms (Agaricus bisporus). Food Hydrocoll. 2017, 72, 202–209. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, R.; Li, W.; Song, J.; Cheng, F. Improved Postharvest Preservation Effects of Pholiota nameko Mushroom by Sodium Alginate–Based Edible Composite Coating. Food Bioprocess Technol. 2019, 12, 587–598. [Google Scholar] [CrossRef]
- Prabha, V.; Barma, R.D.; Singh, R.; Madan, A. Ozone Technology in Food Processing: A Review. Trends Biosci. 2015, 8, 4031–4047. [Google Scholar]
- Akata, I.; Torlak, E.; Erci, F. Efficacy of gaseous ozone for reducing microflora and foodborne pathogens on button mushroom. Postharvest Biol. Technol. 2015, 109, 40–44. [Google Scholar] [CrossRef]
- Watanabe, T.; Tsuchihashi, N.; Takai, Y.; Tanaka, K.; Suzuki, A. Effects of ozone exposure during cultivation of oyster mushroom (Pleurotus ostreatus) on chemical components of the fruit bodies. Nippon Shokuhin Kogyo Gakkaishi 1994, 41, 705–708. [Google Scholar] [CrossRef] [Green Version]
- Anjaly, S.M.; Khanashyam, A.C.; Balasubrahmanyam, B.V.S.; Yadav, B.K. Potentials of Ozone Pre-treatment in Prolonging the Freshness of Oyster Potentials of Ozone Pre-treatment in Prolonging the Freshness of Oyster Mushrooms ( Pleurotus Florida ). Malaysian J. Med. Heal. Sci. 2020, 16, 119–120. [Google Scholar]
- Zalewska, M.; Górska-Horczyczak, E.; Marcinkowska-Lesiak, M. Effect of Applied Ozone Dose, Time of Ozonization, and Storage Time on Selected Physicochemical Characteristics of Mushrooms (Agaricus bisporus). Agriculture 2021, 11, 748. [Google Scholar] [CrossRef]
- Liu, J.; Chang, M.C.; Meng, J.L.; Liu, J.Y.; Cheng, Y.F.; Feng, C.P. Effect of ozone treatment on the quality and enzyme activity of Lentinus edodes during cold storage. J. Food Process. Preserv. 2020, 44, 1–11. [Google Scholar] [CrossRef]
- Rahman, S.; Khan, I.; Oh, D.H. Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.Y.; Kim, N.H.; Jang, I.S.; Jang, S.H.; Lee, S.H.; Hwang, I.G.; Rhee, M.S. Decontamination efficacy of neutral electrolyzed water to eliminate indigenous flora on a large-scale of cabbage and carrot both in the laboratory and on a real processing line. Food Res. Int. 2014, 64, 234–240. [Google Scholar] [CrossRef]
- Aday, M.S. Application of electrolyzed water for improving postharvest quality of mushroom. LWT Food Sci. Technol. 2016, 68, 44–51. [Google Scholar] [CrossRef]
- Ding, T.; Rahman, S.M.E.; Oh, D.H. Inhibitory effects of low concentration electrolyzed water and other sanitizers against foodborne pathogens on oyster mushroom. Food Control 2011, 22, 318–322. [Google Scholar] [CrossRef]
- FDA. CFR—Code of Federal Regulations Title 21. Part 179: Irradiation in the Production, Processing and Handling of Food. Ultraviolet Radiation for the Processing and Treatment of Food. 21CFR179.39. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=50.25 (accessed on 7 April 2021).
- Elmnasser, N.; Guillou, S.; Leroi, F.; Orange, N.; Bakhrouf, A.; Federighi, M. Pulsed-light system as a novel food decontamination technology: A review. Can. J. Microbiol. 2007, 53, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, K.; Shibato, J.; Sameshima, T.; Fukunaga, S.; Isobe, S.; Arihara, K.; Itoh, M. Damage of yeast cells induced by pulsed light irradiation. Int. J. Food Microbiol. 2003, 85, 151–158. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Aguiló-Aguayo, I.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments on quality and antioxidant properties of fresh-cut mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2010, 56, 216–222. [Google Scholar] [CrossRef]
- de São José, J.F.B.; de Andrade, N.J.; Ramos, A.M.; Vanetti, M.C.D.; Stringheta, P.C.; Chaves, J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control 2014, 45, 36–50. [Google Scholar] [CrossRef]
- Guerrero, S.N.; Ferrario, M.; Schenk, M.; Carrillo, M.G. Hurdle Technology Using Ultrasound for Food Preservation. In Ultrasound: Advances in Food Processing and Preservation; Bermudez-Aguirre, D., Ed.; Elsevier Science: London, UK, 2017; pp. 39–99. ISBN 9780128046142. [Google Scholar]
- Cardoso, R.V.C.; Fernandes, Â.; Barreira, J.C.M.; Verde, S.C.; Antonio, A.L.; Gonzaléz-Paramás, A.M.; Barros, L.; Ferreira, I.C.F.R. Effectiveness of gamma and electron beam irradiation as preserving technologies of fresh Agaricus bisporus Portobello: A comparative study. Food Chem. 2019, 278, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, Â.; Antonio, A.L.; Barreira, J.C.M.; Botelho, M.L.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effects of Gamma Irradiation on the Chemical Composition and Antioxidant Activity of Lactarius deliciosus L. Wild Edible Mushroom. Food Bioprocess Technol. 2013, 6, 2895–2903. [Google Scholar] [CrossRef]
- Koorapati, A.; Foley, D.; Pilling, R.; Prakash, A. Electron-beam Irradiation Preserves the Quality of White Button Mushroom (Agaricus bisporus) Slices. J. Food Sci. 2004, 69, 25–29. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barreira, J.C.M.; Günaydi, T.; Alkan, H.; Antonio, A.L.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effect of gamma irradiation and extended storage on selected chemical constituents and antioxidant activities of sliced mushroom. Food Control 2017, 72, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, Â.; Antonio, A.L.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effects of gamma irradiation on physical parameters of Lactarius deliciosus wild edible mushrooms. Postharvest Biol. Technol. 2012, 74, 79–84. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barreira, J.C.M.; Antonio, A.L.; Santos, P.M.P.; Martins, A.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Study of chemical changes and antioxidant activity variation induced by gamma-irradiation on wild mushrooms: Comparative study through principal component analysis. Food Res. Int. 2013, 54, 18–25. [Google Scholar] [CrossRef]
- Jiang, T.; Jahangir, M.M.; Jiang, Z.; Lu, X.; Ying, T. Influence of UV-C treatment on antioxidant capacity, antioxidant enzyme activity and texture of postharvest shiitake (Lentinus edodes) mushrooms during storage. Postharvest Biol. Technol. 2010, 56, 209–215. [Google Scholar] [CrossRef]
- Sławińska, A.; Fornal, E.; Radzki, W.; Skrzypczak, K.; Zalewska-Korona, M.; Michalak-Majewska, M.; Parfieniuk, E.; Stachniuk, A. Study on Vitamin D2 stability in dried mushrooms during drying and storage. Food Chem. 2016, 199, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.A.; Lee, B.H.; Lee, J.S.; Park, H.J. Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J. Agric. Food Chem. 2008, 56, 3671–3674. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Lin, C.P.; Tsai, S.Y. Vitamin D2 content and antioxidant properties of fruit body and mycelia of edible mushrooms by UV-B irradiation. J. Food Compos. Anal. 2015, 42, 38–45. [Google Scholar] [CrossRef]
- Potluri, S.; Sangeetha, K.; Santhosh, R.; Nivas, G.; Mahendran, R. Effect of low-pressure plasma on bamboo rice and its flour. J. Food Process. Preserv. 2018, 42, 1–7. [Google Scholar] [CrossRef]
- Alkawareek, M.Y.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Potential cellular targets and antibacterial efficacy of atmospheric pressure non-thermal plasma. Int. J. Antimicrob. Agents 2014, 43, 154–160. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.H.; Jo, C. Prospective Applications of Cold Plasma for Processing Poultry Products: Benefits, Effects on Quality Attributes, and Limitations. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1292–1309. [Google Scholar] [CrossRef] [Green Version]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.D.; Von Woedtke, T. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Gavahian, M.; Sheu, F.H.; Tsai, M.J.; Chu, Y.H. The effects of dielectric barrier discharge plasma gas and plasma-activated water on texture, color, and bacterial characteristics of shiitake mushroom. J. Food Process. Preserv. 2020, 44, 1–9. [Google Scholar] [CrossRef]
- Misra, N.N.; Koubaa, M.; Roohinejad, S.; Juliano, P.; Alpas, H.; Inácio, R.S.; Saraiva, J.A.; Barba, F.J. Landmarks in the historical development of twenty first century food processing technologies. Food Res. Int. 2017, 97, 318–339. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Koubaa, M.; do Prado-Silva, L.; Orlien, V.; Sant’Ana, A.d.S. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Technol. 2017, 66, 20–35. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Ranganathan, K.; Subramanian, V.; Shanmugam, N. Effect of Thermal and Nonthermal Processing on Textural Quality of Plant Tissues. Crit. Rev. Food Sci. Nutr. 2016, 56, 2665–2694. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Farkas, D.; Turek, E.J. Preserving foods through high-pressure processing. Food Technol. 2008, 62, 32–38. [Google Scholar]
- Whitaker, J.R. Enzymes: Monitors of food stability and quality. Trends Food Sci. Technol. 1991, 2, 94–97. [Google Scholar] [CrossRef]
- Yang, H.; Yu, X.R.; Qian, D.K.; Liu, L.J.; Qi, X.Y. Effect of high hydrostatic pressure treatment on the quality of Pleurotus eryngii. Mod. Food Sci. Technol. 2014, 30, 164–169. [Google Scholar] [CrossRef]
- Yi, J.; Dong, P.; Ding, G.; Wang, T.-T.; Hu, X.S.; Zhang, Y. Study on effect of high hydrostatic pressure on microbial inactivation and inactivation kinetics in mushroom. Sci. Technol. Food Ind. 2012, 9, 78–81. [Google Scholar]
- Matser, A.M.; Knott, E.R.; Teunissen, P.G.M.; Bartels, P.V. Effects of high isostatic pressure on mushrooms. J. Food Eng. 2000, 45, 11–16. [Google Scholar] [CrossRef]
- Lagnika, C.; Zhang, M.; Mothibe, K.J. Effects of ultrasound and high pressure argon on physico-chemical properties of white mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Biol. Technol. 2013, 82, 87–94. [Google Scholar] [CrossRef]
- Gomes, M.R.A.; Ledward, D.A. Effect of high-pressure treatment on the activity of some polyphenoloxidases. Food Chem. 1996, 56, 1–5. [Google Scholar] [CrossRef]
- Sun, N.K.; Song, K.B. Effect of nonthermal treatment on the molecular properties of mushroom polyphenoloxidase. J. Food Sci. 2003, 68, 1639–1643. [Google Scholar] [CrossRef]
- Yi, J.; Jiang, B.; Zhang, Z.; Liao, X.; Zhang, Y.; Hu, X. Effect of ultrahigh hydrostatic pressure on the activity and structure of mushroom (Agaricus bisporus) polyphenoloxidase. J. Agric. Food Chem. 2012, 60, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Khair, A.; Begum, F.; Chowdhury, K.; Karim, R. Effect of respiratory gases (O2; CO2) on shelf-life of fresh oyster mushrooms packaged with different sealable polymeric materials. Bangladesh J. Sci. Ind. Res. 2015, 50, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Dhalsamant, K.; Dash, S.K.; Bal, L.M.; Panda, M.K. Effect of perforation mediated MAP on shelf life of mushroom (Volvariella volvacea). Sci. Hortic. 2015, 189, 41–50. [Google Scholar] [CrossRef]
- Jiang, T.; Zheng, X.; Li, J.; Jing, G.; Cai, L.; Ying, T. Integrated application of nitric oxide and modified atmosphere packaging to improve quality retention of button mushroom (Agaricus bisporus). Food Chem. 2011, 126, 1693–1699. [Google Scholar] [CrossRef]
- Ahn, B.-K.; Noh-Hyum, P. Mushroom (Agaricus bisporus) pre-packaging by the rice straw pulp tray. Korean J. Food Sci. Technol. 1995, 27, 353–357. [Google Scholar]
- Qin, Y.; Liu, D.; Wu, Y.; Yuan, M.; Li, L.; Yang, J. Effect of PLA/PCL/cinnamaldehyde antimicrobial packaging on physicochemical and microbial quality of button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2015, 99, 73–79. [Google Scholar] [CrossRef]
- Roy, S.; Anantheswaran, R.C.; Beelman, R.B. Fresh mushroom quality as affected by modified atmosphere packaging. J. Food Sci. 1995, 60, 334–340. [Google Scholar] [CrossRef]
- Charles, F.; Guillaume, C.; Gontard, N. Effect of passive and active modified atmosphere packaging on quality changes of fresh endives. Postharvest Biol. Technol. 2008, 48, 22–29. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Opara, U.L. Modelling approaches for designing and evaluating the performance of modified atmosphere packaging (MAP) systems for fresh produce: A review. Food Packag. Shelf Life 2016, 10, 1–15. [Google Scholar] [CrossRef]
- Lopez-Briones, G.L.; Varaquaux, P.; Chambroy, Y.; Bouquant, J.; Bureau, G.; Pascat, B. Storage of common mushroom under controlled atmospheres. Int. J. Food Sci. Technol. 1992, 27, 493–505. [Google Scholar] [CrossRef]
- Oliveira, F.; Sousa-Gallagher, M.J.; Mahajan, P.V.; Teixeira, J.A. Development of shelf-life kinetic model for modified atmosphere packaging of fresh sliced mushrooms. J. Food Eng. 2012, 111, 466–473. [Google Scholar] [CrossRef] [Green Version]
- Andrés, A.I.; Timón, M.L.; Molina, G.; González, N.; Petrón, M.J. Effect of MAP storage on chemical, physical and sensory characteristics of “níscalos” (Lactarius deliciosus). Food Packag. Shelf Life 2014, 1, 179–189. [Google Scholar] [CrossRef]
- Li, Y.; Ishikawa, Y.; Satake, T.; Kitazawa, H.; Qiu, X.; Rungchang, S. Effect of active modified atmosphere packaging with different initial gas compositions on nutritional compounds of shiitake mushrooms (Lentinus edodes). Postharvest Biol. Technol. 2014, 92, 107–113. [Google Scholar] [CrossRef]
- Halachmy, I.B.; Mannheim, C.H. Modified atmosphere packaging of fresh mushrooms. Packag. Technol. Sci. 1991, 4, 279–286. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Hu, H.; Sun, Y.; Wang, Y.; Zhao, Y. High carbon dioxide and low oxygen storage effects on reactive oxygen species metabolism in Pleurotus eryngii. Postharvest Biol. Technol. 2013, 85, 141–146. [Google Scholar] [CrossRef]
- Rux, G.; Mahajan, P.V.; Linke, M.; Pant, A.; Sängerlaub, S.; Caleb, O.J.; Geyer, M. Humidity-Regulating Trays: Moisture Absorption Kinetics and Applications for Fresh Produce Packaging. Food Bioprocess Technol. 2016, 9, 709–716. [Google Scholar] [CrossRef]
- Kim, K.M.; Ko, J.A.; Lee, J.S.; Park, H.J.; Hanna, M.A. Effect of modified atmosphere packaging on the shelf-life of coated, whole and sliced mushrooms. LWT Food Sci. Technol. 2006, 39, 365–372. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Del-Toro-Sánchez, L.; Alvarez-Parrilla, E.; González-Aguilar, G.A. High relative humidity in-package of fresh-cut fruits and vegetables: Advantage or disadvantage considering microbiological problems and antimicrobial delivering systems? J. Food Sci. 2008, 73, R41–R47. [Google Scholar] [CrossRef] [PubMed]
- Linke, M.; Geyer, M. Condensation dynamics in plastic film packaging of fruit and vegetables. J. Food Eng. 2013, 116, 144–154. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Oliveira, F.A.R.; Montanez, J.C.; Frias, J. Development of user-friendly software for design of modified atmosphere packaging for fresh and fresh-cut produce. Innov. Food Sci. Emerg. Technol. 2007, 8, 84–92. [Google Scholar] [CrossRef]
- Simón, A.; González-Fandos, E.; Tobar, V. The sensory and microbiological quality of fresh sliced mushroom (Agaricus bisporus L.) packaged in modified atmospheres. Int. J. Food Sci. Technol. 2005, 40, 943–952. [Google Scholar] [CrossRef]
- Villaescusa, R.; Gil, M.I. Quality improvement of Pleurotus mushrooms by modified atmosphere packaging and moisture absorbers. Postharvest Biol. Technol. 2003, 28, 169–179. [Google Scholar] [CrossRef]
- Singh, P.; Wani, A.A.; Saengerlaub, S. Active packaging of food products: Recent trends. Nutr. Food Sci. 2011, 41, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Duan, Z.; Xing, Z.; Shao, Y.; Zhao, X. Effect of electron beam irradiation on postharvest quality and selected enzyme activities of the white button mushroom, agaricus bisporus. J. Agric. Food Chem. 2010, 58, 9617–9621. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, S.; Priyadarshini, S.R.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jang, N.Y.; Won, K. New pressure-activated compartmented oxygen indicator for intelligent food packaging. Int. J. Food Sci. Technol. 2014, 49, 650–654. [Google Scholar] [CrossRef]
- Pei, Z.; Yang, Y.; Chen, Q.; Wei, Y.; Ji, Y. Regional Shape Control of Strategically Assembled Multishape Memory Vitrimers. Adv. Mater. 2016, 28, 156–160. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Nerín, C. Performance of an active paper based on cinnamon essential oil in mushrooms quality. Food Chem. 2015, 170, 30–36. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhang, X.; Kan, J.; Jin, C. Effect of gallic acid grafted chitosan film packaging on the postharvest quality of white button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 147, 39–47. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Sun, Y.; Wang, X.; Li, L. Combined antioxidant and sensory effects of active chitosan/zein film containing α-tocopherol on Agaricus bisporus. Food Packag. Shelf Life 2020, 24, 100470. [Google Scholar] [CrossRef]
- Ni, X.; Yu, J.; Shao, P.; Yu, J.; Chen, H.; Gao, H. Preservation of Agaricus bisporus freshness with using innovative ethylene manipulating active packaging paper. Food Chem. 2020, 345, 128757. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gaikwad, K.K.; Lee, M.; Lee, Y.S. Thermally buffered corrugated packaging for preserving the postharvest freshness of mushrooms (Agaricus bispours). J. Food Eng. 2018, 216, 11–19. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Bodenhamer, W.T.; Jackowski, G.; Davies, E. Surface binding of an inmunoglobulin to a flexible polymer using a water soluble varnish matrix. U.S. Patent 6692973, 17 February 2004. [Google Scholar]
- Vanderroost, M.; Ragaert, P.; Devlieghere, F.; De Meulenaer, B. Intelligent food packaging: The next generation. Trends Food Sci. Technol. 2014, 39, 47–62. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; De Leon-Zapata, M.A.; Alvarez-Perez, O.B.; Torres-León, C.; Nieto-Oropeza, D.E.; Ventura-Sobrevilla, J.M.; Aguilar, M.A.; Ruelas-Chacón, X.; Rojas, R.; Ramos-Aguiñaga, M.E.; et al. Basic and Applied Concepts of Edible Packaging for Foods. In Food Packaging and Preservation; Elsevier Inc.: London, UK, 2018; pp. 1–61. ISBN 9780128115169. [Google Scholar]
- Pooja Saklani, P.S.; Nath, S.; Kishor Das, S.; Singh, S.M. A review of edible packaging for foods. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2885–2895. [Google Scholar] [CrossRef]
- Licciardello, F. Packaging, blessing in disguise. Review on its diverse contribution to food sustainability. Trends Food Sci. Technol. 2017, 65, 32–39. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M. Nanoedible films for food packaging: A review. J. Mater. Sci. 2019, 54, 12290–12318. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Ecological and Solidarity Transition. French National Pact on Plastic Packaging. Available online: https://www.gouvernement.fr/sites/default/files/locale/piece-jointe/2019/06/11_french-national-_pact-on-plastic-packaging_pdf_0.pdf (accessed on 12 April 2021).
- Avérous, L.; Pollet, E. Biodegradable Polymers. In Envioronmental Silictae Nano-Biocomposites; Avérous, L., Pollet, E., Eds.; Springer New York: New York, NY, USA, 2012; pp. 13–39. ISBN 9781447141082. [Google Scholar]

| Materials | Film Material | Process Parameters | Results | References |
|---|---|---|---|---|
| Synthetic | Expanded polystyrene. | Polystyrene tray wrapped with PVC film. Storage temperature of fresh mushrooms (Pleurotus ostreatus): 5 ± 1 °C. | Increased shelf life to 12 days. | [145] |
| Mediated perforation low-density polyethylene film. | Film thickness: 95 μm Perforation diameter: 0.45 mm. Number of perforations in container: 0, 20, 40, 60. Storage temperature of fresh mushrooms (Volvariella volvacea): 12 (±1) °C. Pretreatment: half of the samples were treated with 0.5% CaCl2. | The atmosphere mediated by the perforation increased the shelf life of the mushroom to 6 days. Mushroom firmness was better preserved with packages with 20 and 40 perforations. There were no significant differences in total protein content between the 20- and 40-perforationg groups, but total phenolic content, antioxidants, and bacterial count were best in the pretreated samples stored in containers with 40 perforations. | [146] | |
| Biaxially oriented polypropylene bags. | Pretreatments: immersion in different concentrations of 2,2′-(hydroxynitrosohydrazino)-bisethanamine (DETANO) for 10 min Storage temperature of fresh mushrooms (Agaricus bisporus): 4 °C. | Treatment with 1 mM DETANOmaintained a high level of firmness and delayed browning and cap opening. DETANO in combination with modified atmosphere extended storage life up to 12 days. | [147] | |
| Low-density polyethylene Film thickness: 0.04 mm. | Storage temperature of fresh mushrooms (Agaricus bisporus): 4 °C. Relative humidity: 85%. | Elevated CO2 reduced the browning of fungi and increased total phenolic content and activity and total antioxidant activity, extending the shelf life of the button mushroom. | [56] | |
| Biodegradable | Prepackaging trays from agricultural waste fibers. | Prepackaging trays made from rice-industry waste for fresh mushrooms (Agaricus bisporus) at a temperature of 4 °C and 25 °C. | The weight losses of the mushrooms remained below 5%, and the Hunter L values were above 76 at 4 °C, while the mushrooms packed in trays of rice pulp showed a greater lightness than the ones packed in polystyrene trays stored at 25 °C. | [148] |
| Films of a mixture of poly (lactic acid) (PLA) and poly(ε-caprolactone) (PCL) with different cinnamaldehyde concentrations. | Storage temperature of fresh mushrooms (Agaricus bisporus): 4 ± 1 °C and relative humidity: 85%. | The greatest weight loss of the mushrooms packed with PLA and PCL was 3.08% at the end of storage. The level of CO2 inside the PLA and PCL films with cinnamaldehyde was lower than that of the PLA and PCL films without cinnamaldehyde, but the level of O2 in these two types of films was similar. | [149] |
| Elements | Function/Results | References |
|---|---|---|
| Sulfur dioxide, green tea extract, cinnamon essential oil, purple carrot extract. These active agents were incorporated on filter paper as an active label and on polyethylene terephthalate film. | Active agents incorporated to extend the shelf life of fresh mushrooms. Active packaging exhibited antioxidant properties without being in direct contact with the mushrooms while maintaining the white color. | [54] |
| Macroperforated polyethylene terephthalate trays covered with cinnamon essential oil and cinnamaldehyde active papers. | Active paper had elements that preserved the mushrooms against oxidation, inhibiting tyrosinase to extend their shelf life. | [175] |
| Film with the addition of active agents such as grafted chitosan and gallic acid and polyethylene films. | Film used as a new active packaging material for the conservation of Agaricus bisporus, promoting the maintenance of its postharvest quality. There was a lower respiration rate and a lower degree of browning but a higher antioxidant status than those packed with chitosan and polyethylene films. | [176] |
| Chitosan/zein (tocopherol) films as packaging for mushrooms. | Active film used to extend the shelf life of Agaricus bisporus, which improve the deterioration of the quality of the mushrooms, reducing weight loss, maintaining firmness, and decreasing browning. | [177] |
| Packaging paper prepared with 1-methylcyclopropene and potassium permanganate. | Functional paper used for the elimination of ethylene and to increase the storage time of the mushroom Agaricus bisporus; delayed the softening, browning, and weight loss of mushrooms during the storage period. It had an acceptable quality, with weight loss of 4.66%, firmness of 0.79 kgf, lightness of 86.11, and browning index of 36.18 after 6 days of storage. | [178] |
| Polytextile, thermoregulatory material coated with microencapsulated paraffin melamine powder. | Intelligent packaging used for temperature regulation (5 °C) of mushrooms. Quality parameters were kept within the acceptable limits. | [179] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellanos-Reyes, K.; Villalobos-Carvajal, R.; Beldarrain-Iznaga, T. Fresh Mushroom Preservation Techniques. Foods 2021, 10, 2126. https://doi.org/10.3390/foods10092126
Castellanos-Reyes K, Villalobos-Carvajal R, Beldarrain-Iznaga T. Fresh Mushroom Preservation Techniques. Foods. 2021; 10(9):2126. https://doi.org/10.3390/foods10092126
Chicago/Turabian StyleCastellanos-Reyes, Katy, Ricardo Villalobos-Carvajal, and Tatiana Beldarrain-Iznaga. 2021. "Fresh Mushroom Preservation Techniques" Foods 10, no. 9: 2126. https://doi.org/10.3390/foods10092126