Reducing the Negative Effect on White Wine Chromatic Characteristics Due to the Oxygen Exposure during Transportation by the Deoxygenation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wine Samples
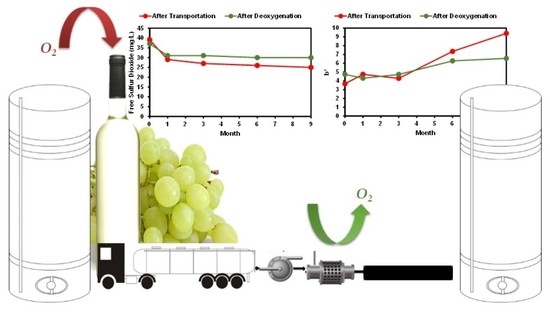
2.2. Experimental Design
2.3. Analysis of Conventional Enological Parameters
2.4. Total Phenols, Non-Flavonoid, and Flavonoid Phenols
2.5. Total Tannins
2.6. Gelatin Index
2.7. Color and Chromatic Characteristics
2.8. Acetaldehyde
2.9. Dissolved Oxygen
2.10. Kinetic Analysis—Kinetic Modeling
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Oxygen Uptake during Transportation and Deoxygenation on Wine Quality
3.1.1. Oxygen, Sulfur Dioxide, Acetaldehyde, Volatile Acidity Levels during Storage
3.1.2. Color and Chromatic Characteristic Evolution during Storage
3.1.3. Effect of Oxygen Content on the Phenolic Composition Evolution during Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godden, P.; Lattey, K.; Francis, L.; Gishen, M.; Cowey, G.; Holdstock, M.; Robinson, E.; Waters, E.; Skouroumounis, G.; Sefton, M.; et al. Towards offering wine to the consumer in optimal condition; the wine, the closures and other packaging variables. A review of AWRI research examining the changes that occur in wine after bottling. Wine Ind. J. 2005, 20, 20–30. [Google Scholar]
- Day, M.P.; Schmidt, S.A.; Smith, P.A.; Wilkes, E.N. Use and impact of oxygen during winemaking. Aust. J. Grape Wine Res. 2015, 21, 693–704. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Siuta, M. The Impact of Oxygen at Various Stages of Vinification on the Chemical Composition and the Antioxidant and Sensory Properties of White and Red Wines. Int. J. Food Sci. 2020, 2020, 7902974. [Google Scholar] [CrossRef] [Green Version]
- Caillé, S.; Samson, A.; Wirth, J.; Diéval, J.; Vidal, S.; Cheynier, V. Sensory characteristics changes of red Grenache wines submitted to different oxygen exposures preé and post bottling. Anal. Chim. Acta 2010, 310, 35–36. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.C.; Dufourq, T.; Moutounet, M. Les apports d’oxygène au cours des traitements des vins. Revue Française d’Oenologie 2001, 190, 24–51. [Google Scholar]
- Castellari, M.; Simonato, B.; Tornielli, G.B.; Spinelli, P.; Ferrarini, R. Effects of different enological treatments on dissolved oxygen in wines. Ital. J. Food Sci. 2004, 16, 387–396. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. The chemistry of wine stabilization and treatments. In Handbook of Enology; John Wiley & Sons, Ltd.: Chichester, UK, 2006; Volume 2. [Google Scholar]
- Du Toit, W.J.; Marais, J.; Pretorius, I.S.; du Toit, M. Oxygen in Must and Wine: A review. S. Afr. J. Enol. Vitic. 2006, 27, 76–94. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, C.; du Toit, W.J. Sauvignon blanc wine: Contribution of ageing and oxygen on aromatic and non-aromatic compounds and sensory composition—A review. S. Afr. J. Enol. Vitic. 2015, 36, 347–365. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Kramling, T.E. Browning of white wines and accelerated test for browning capacity. Am. J. Enol. Vitic. 1976, 27, 157–160. [Google Scholar]
- Singleton, V.L.; Trousdale, E.; Zaya, J. Oxidation of wines. I. Young white wines periodically exposed to air. Am. J. Enol. Vitic. 1979, 30, 49–54. [Google Scholar]
- Escudero, A.; Hernandez-Orte, P.; Cacho, J.E.; Ferreira, V. Clues about the role of methional as a character impact odorant of some oxidized wines. J. Agric. Food Chem. 2000, 48, 4268–4272. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Cacho, J.; Ferreira, V. Isolation and identification of odourants generated in wine during its oxidation: A gas-chromatography-olfactometry study. Eur. Food Res. Technol. 2000, 211, 105–110. [Google Scholar] [CrossRef]
- Gonzalez-Cartagena, L.; Perez-Zuniga, F.J.; Abad, F.B. Interactions of some environmental and chemical parameters affecting the colour attribute of wine. Am. J. Enol. Vitic. 1994, 45, 43–48. [Google Scholar]
- Sioumis, N.; Kallithraka, S.; Tsoutsouras, E.; Makris, D.P.; Kefalas, P. Browning development in white wines: Dependence on compositional parameters and impact on antioxidant characteristics. Eur. Food Res. Technol. 2005, 220, 326–330. [Google Scholar] [CrossRef]
- Salacha, M.-I.; Kallithraka, S.; Tzourou, I. Browning of white wines: Correlation with antioxidant characteristics, total polyphenolic composition and flavanol content. Int. J. Food Sci. Technol. 2008, 43, 1073–1077. [Google Scholar] [CrossRef]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Singleton, V.L. Oxygen with phenols and related reactions in musts, wines and model systems: Observation and practical implications. Am. J. Enol. Vitic. 1987, 38, 69–73. [Google Scholar]
- Somers, T.C.; Evans, M.E. Evolution of red wine. Ambient influences on color composition during early maturation. Vitis 1986, 25, 31–39. [Google Scholar]
- Lopes, P.; Saucier, C.; Glories, Y. Nondestructive colorimetric method to determine the oxygen diffusion rate through closures used in winemaking. J. Agric. Food Chem. 2005, 53, 6967–6973. [Google Scholar] [CrossRef]
- Barril, C.; Clark, A.C.; Scollary, G.R. Chemistry of ascorbic acid and sulfur dioxide as an antioxidant system relevant to white wine. Anal. Chim. Acta 2012, 732, 186–193. [Google Scholar] [CrossRef]
- Wildenradt, H.L.; Singleton, V.L. The production of aldehydes as a result of oxidation of polyphenolic compounds and its relation to wine aging. Am. J. Enol. Vit. 1974, 25, 119–126. [Google Scholar]
- Danilewicz, J.C. Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: Central role of copper and iron. Am. J. Enol. Vitic. 2003, 54, 73–85. [Google Scholar]
- Carrascon, V.; Vallverdu-Queralt, A.; Meudec, E.; Sommerer, N.; Fernandez-Zurbano, P.; Ferreira, V. The kinetics of oxygen and SO2 consumption by red wines. What do they tell about oxidation mechanisms and about changes in wine composition? Food Chem. 2018, 241, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Springer Science & Business Media: Berlin, Germany, 1996. [Google Scholar]
- Danilewicz, J.C. Reaction of Oxygen and Sulfite in Wine. Am. J. Enol. Vitic. 2015, 67, 13–17. [Google Scholar] [CrossRef]
- Lopes, P.; Saucier, C.; Teissedre, P.; Glories, Y. Impact of storage position on oxygen ingress trough different closures in to wines bottles. J. Agric. Food Chem. 2006, 54, 6741–6746. [Google Scholar] [CrossRef] [PubMed]
- OIV. Organisation International de la Vigne et du Vin; Code International des Pratiques Œnologiques: Paris, France, 2021. [Google Scholar]
- Kramling, T.E.; Singleton, V.L. An estimate of the nonflavonoid phenols in wines. Am. J. Enol. Vitic. 1969, 20, 86–92. [Google Scholar]
- Ribéreau-Gayon, P.; Stonestreet, E. Dosage des tanins du vin rouge et détermination de leur structure. Chim. Anal. 1966, 4, 188–196. [Google Scholar]
- Glories, Y. La coleur des vins rouges, 2eme Partier. Mesure, origine et interpretation. Connaiss. Vigne Vin 1984, 18, 253–271. [Google Scholar]
- Spagna, G.; Pifferi, P.G.; Rangoni, C.; Mattivi, F.; Nicolini, G.; Palmonari, R. The stabilization of white wines by adsorption of phenolic compounds on chitin and chitosan. Food Res. Int. 1996, 29, 241–248. [Google Scholar] [CrossRef]
- Martins, R.C.; Lopes, V.V.; Vicente, A.A.; Teixeira, J.A. Computational Shelf-Life Dating: Complex Systems Approaches to Food Quality and Safety. Food Bioproc. Tech. 2008, 1, 207–222. [Google Scholar] [CrossRef] [Green Version]
- Doran, P.M. Bioprocess Engineering Principles, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 379–444. ISBN 9780122208515. [Google Scholar]
- Jacobs, D. Effect of dissolved oxygen on free sulphur dioxide in red wines. Am. J. Enol. Vitic. 1976, 27, 1–7. [Google Scholar]
- Danilewicz, J.C.; Seccombe, J.T.; Whelan, J. Mechanism of Interaction of Polyphenols, Oxygen and Sulfur Dioxide in Model Wine and Wine. Am. J. Enol. Vitic. 2008, 59, 128–136. [Google Scholar]
- Wirth, J.; Morel-Salmi, C.; Souquet, J.M.; Dieval, J.B.; Aagaard, O.; Vidal, S.; Fulcrand, H.; Cheynier, V. The impact of oxygen exposure before and after bottling on the polyphenolic composition of red wines. Food Chem. 2010, 123, 107–116. [Google Scholar] [CrossRef]
- Carrascon, V.; Fernandez-Zurbano, P.; Bueno, M.; Ferreira, V. Oxygen consumption by red wines. Part II: Differential effects on color and chemical composition caused by oxygen taken in different sulfur dioxide-related oxidation contexts. J. Agric. Food Chem. 2015, 63, 10938–10947. [Google Scholar] [CrossRef] [Green Version]
- Carrascon, V.; Bueno, M.; Fernandez-Zurbano, P.; Ferreira, V. Oxygen and SO2 consumption rates in white and rosé wines: Relationship with and effects on wine chemical composition. J. Agric. Food Chem. 2017, 65, 9488–9495. [Google Scholar] [CrossRef] [Green Version]
- Fabre, S. Destination de l’oxygène consommé par le vin. Rev. Enol. 1994, 71, 23–26. [Google Scholar]
- Escudero, A.; Asenio, E.; Cacho, J.; Ferreira, V. Sensory and chemical changes of young wines stored under oxygen. An assessment of the role played by aldehydes and some others important odorants. Food Chem. 2002, 77, 325–331. [Google Scholar] [CrossRef]
- Carlton, W.K.; Gump, B.; Fuselgang, K.; Hasson, A.S. Monitoring Acetaldehyde Concentrations during Micro-oxygenation of Red Wine by Headspace Solid-Phase Microextration with On-Fiber Derivatization. J. Agric. Food Chem. 2007, 55, 5620–5625. [Google Scholar] [CrossRef]
- Dias, D.A.; Smith, T.A.; Ghiggino, K.P.; Scollary, G.R. The role of light, temperature and wine bottle colour on pigment enhancement in white wine. Food Chem. 2012, 15, 2934–2941. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Interaction of sulfur dioxide, polyphenols and oxygen in a winemodel system: Central role of iron and copper. Am. J. Enol. Vit. 2007, 58, 53–60. [Google Scholar]
- Barroso, C.G.; Lópes-Sánchez, L.; Otero, J.C.; Cela, R.; Pérez-Bustament, J.A. Studies on the browning of fino sherry and its relation to phenolic compounds. Z. Lebensm. Unters. Forsch 1989, 189, 322–325. [Google Scholar] [CrossRef]
- de Beer, D.; Joubert, J.; Manley, M. Effect of oxygenation during maturation on phenolic composition, total antioxidant capacity, colour and sensory quality of pinotage wine. S. Afr. J. Enol. Vitic. 2008, 29, 13–25. [Google Scholar]
- Pérez-Magariño, S.; González-San José, M.L. Influence of commercial pectolytic preparations on the composition and storage evolution of Albillo white wines. Int. J. Food Sci. 2001, 36, 789–796. [Google Scholar] [CrossRef]
- Somers, T.C. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
| Volatile Acidity (g/L Expressed as Acetic Acid) | Free Sulfur Dioxide (mg/L) | Dissolved Oxygen (mg/L) | Acetaldehyde (mg/L) | Volatile Acidity (g/L Expressed as Acetic acid) | Free Sulfur Dioxide (mg/L) | Dissolved Oxygen (mg/L) | Acetaldehyde (mg/L) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Loureiro Monovarietal Wine | Blend Wine | |||||||||
| Initial time | T0L0 | 0.28 ± 0.01 a | 44 ± 1 a | 1.50 ± 0.04 a | 29.8 ± 1.0 a | T0B0 | 0.31 ± 0.01 a | 41 ± 1 a | 1.98 ± 0.09 a | 34.5 ± 1.3 a |
| T0L1 | 0.29 ± 0.01 a | 39 ± 1 b | 4.21 ± 0.04 b | 29.3 ± 0.5 a | T0B1 | 0.34 ± 0.02 a | 39 ± 0 a | 4.30 ± 0.01 b | 33.5 ± 0.6 a | |
| T0L2 | 0.28 ± 0.01 a | 37 ± 1 c | 0.09 ± 0.01 c | 29.0 ± 0.0 a | T0B2 | 0.35 ± 0.00 a | 37 ± 0 a | 0.09 ± 0.01 c | 32.3 ± 0.5 a | |
| First month | T1L0 | 0.29 ± 0.01 a | 35 ± 1 d | 0.36 ± 0.02 d | 31.0 ± 0.8 a | T1B0 | 0.33 ± 0.02 a | 36 ± 1 a | 1.23 ± 0.02 d | 37.3 ± 0.5 b |
| T1L1 | 0.29 ± 0.00 a | 31 ± 1 e | 0.66 ± 0.03 e | 28.3 ± 0.5 a | T1B1 | 0.38 ± 0.01 a | 29 ± 1 b | 1.11 ± 0.01 e | 34.8 ± 0.5 a | |
| T1L2 | 0.28 ± 0.01 a | 32 ± 0 e | 0.07 ± 0.01 c | 29.0 ± 0.0 a | T1B2 | 0.37 ± 0.02 a | 31 ± 1 c | 0.07 ± 0.02 c | 36.8 ± 1.5 b | |
| Third month | T3L0 | 0.31 ± 0.01 a | 26 ± 1 f | 0.25 ± 0.03 f | 31.8 ± 0.5 a | T3B0 | 0.35 ± 0.01 a | 34 ± 2 c | 0.98 ± 0.01 f | 38.5 ± 1.3 b |
| T3L1 | 0.34 ± 0.01 b | 25 ± 0 f | 0.42 ± 0.02 g | 33.0 ± 1.0 a | T3B1 | 0.44 ± 0.01 b | 27 ± 1 b | 0.81 ± 0.01 g | 34.5 ± 3.7 a | |
| T3L2 | 0.31 ± 0.02 a | 30 ± 0 e | 0.08 ± 0.01 c | 30.3 ± 0.1 a | T3B2 | 0.37 ± 0.01 a | 31 ± 1 c | 0.05 ± 0.01 c | 36.8 ± 1.7 b | |
| Sixth month | T6L0 | 0.34 ± 0.01 b | 22 ± 0 g | 0.15 ± 0.01 c | 29.8 ± 1.0 a | T6B0 | 0.42 ± 0.01 b | 32 ± 1 c | 0.91 ± 0.01 f | 45.5 ± 1.3 c |
| T6L1 | 0.37 ± 0.01 c | 20 ± 1 g | 0.27 ± 0.01 f | 30.0 ± 0.0 a | T6B1 | 0.45 ± 0.01 b | 26 ± 1 b | 0.42 ± 0.04 h | 42.3 ± 0.5 c | |
| T6L2 | 0.33 ± 0.01 a | 26 ± 0 f | 0.08 ± 0.01 c | 31.0 ± 0.0 a | T6B2 | 0.43 ± 0.01 b | 30 ± 1 c | 0.03 ± 0.01 c | 31.0 ± 0.0 a | |
| Ninth month | T9L0 | 0.39 ± 0.01 c | 18 ± 0 g | 0.12 ± 0.01 c | 29.5 ± 0.6 a | T9B0 | 0.44 ± 0.01 b | 31 ± 1 c | 0.58 ± 0.01 i | 46.3 ± 1.0 d |
| T9L1 | 0.37 ± 0.01 c | 17 ± 1 g | 0.27 ± 0.02 f | 28.7 ± 0.2 a | T9B1 | 0.45 ± 0.01 b | 25 ± 1 b | 0.26 ± 0.01 j | 42.3 ± 0.1 c | |
| T9L2 | 0.34 ± 0.01 b | 21 ± 1 g | 0.07 ± 0.01 c | 30.1 ± 0.1 a | T9B2 | 0.44 ± 0.01 b | 30 ± 0 c | 0.02 ± 0.01 c | 33.0 ± 0.8 a | |
| Wine | Oxygen—Pseudo 2nd Order | k | r | p-Value < |
|---|---|---|---|---|
| Loureiro (L) | Stage 0 | 0.825 ± 0.036 | 0.983 | 0.0001 |
| Stage 1 | 0.372 ± 0.039 | 0.915 | 0.0001 | |
| Stage 2 | - | - | - | |
| Blend (B) | Stage 0 | 0.115 ± 0.008 | 0.955 | 0.0001 |
| Stage 1 | 0.377 ± 0.014 | 0.988 | 0.0001 | |
| Stage 2 | - | - | - | |
| Free sulfur dioxide—Pseudo 2nd Order | ||||
| Loureiro (L) | Stage 0 | 0.00353 ± 0.00014 a | 0.987 | 0.0001 |
| Stage 1 | 0.00325 ± 0.00024 a | 0.955 | 0.0001 | |
| Stage 2 | 0.00218 ± 0.00010 | 0.980 | 0.0001 | |
| Blend (B) | Stage 0 | 0.00081 ± 0.00011 a,b | 0.872 | 0.0001 |
| Stage 1 | 0.00118 ± 0.00024 a | 0.760 | 0.0001 | |
| Stage 2 | 0.000510 ± 0.00012 b | 0.704 | 0.0005 | |
| Volatile Acidity—Pseudo Zero-Order | ||||
| Loureiro (L) | Stage 0 | 0.0116 ± 0.0007 a | 0.969 | 0.0001 |
| Stage 1 | 0.0107 ± 0.0011 a | 0.911 | 0.0001 | |
| Stage 2 | 0.00696 ± 0.00082 | 0.895 | 0.0001 | |
| Blend (B) | Stage 0 | 0.0157 ± 0.0010 | 0.967 | 0.0001 |
| Stage 1 | 0.0109 ± 0.0017 a | 0.834 | 0.0001 | |
| Stage 2 | 0.0105 ± 0.0011 a | 0.913 | 0.0001 |
| Color 1 (A420 nm) | L * | a * | b * | ΔE * | Color 1 (A420 nm) | L * (%) | a * | b * | ΔE * | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loureiro monovarietal wine | Blend wine | |||||||||||
| Initial time | T0L0 | 0.086 ± 0.002 a | 99.0 ± 0.0 a | −2.09 ± 0.03 a | 6.57 ± 0.07 a | T0B0 | 0.128 ± 0.001 a | 91.3 ± 0.0 a | 0.09 ± 0.01 a | 3.99 ± 0.03 a | ||
| T0L1 | 0.085 ± 0.001 a | 97.9 ± 0.0 b | −2.79 ± 0.00 a | 6.65 ± 0.00 a | 1.70 ± 0.11 | T0B1 | 0.128 ± 0.010 a | 90.0 ± 0.2 b | 0.30 ± 0.12 b | 3.67 ± 0.02 a | 1.37 ± 0.02 | |
| T0L2 | 0.080 ± 0.001 a | 97.2 ± 0.0 b | −2.80 ± 0.01 a | 6.84 ± 0.04 a | 1.87 ± 0.04 | T0B2 | 0.128 ± 0.001 a | 96.3 ± 0.0 c | −2.28 ± 0.01 c | 4.74 ± 0.01 b | 1.86 ± 0.02 | |
| First month | T1L0 | 0.089 ± 0.004 a | 98.9 ± 0.0 c | −1.81 ± 0.05 b | 7.17 ± 0.01 a | T1B0 | 0.150 ± 0.004 a | 99.3 ± 0.1 d | −1.58 ± 0.01 d | 4.09 ± 0.02 a | ||
| T1L1 | 0.092 ± 0.002 a | 98.9 ± 0.0 c | −2.04 ± 0.00 c | 7.26 ± 0.05 b | 1.98 ± 0.21 | T1B1 | 0.142 ± 0.003 a | 99.6 ± 0.0 d | −1.61 ± 0.03 d | 4.97 ± 0.02 b | 0.34 ± 0.06 | |
| T1L2 | 0.085 ± 0.001 a | 98.9 ± 0.0 c | −1.76 ± 0.04 b | 7.34 ± 0.20 b | 1.86 ± 0.01 | T1B2 | 0.135 ± 0.001 a | 99.1 ± 0.3 d | −1.59 ± 0.03 d | 4.30 ± 0.01 c | 0.45 ± 0.27 | |
| Third month | T3L0 | 0.126 ± 0.014 b | 97.9 ± 0.1 b | −1.94 ± 0.03 c | 7.68 ± 0.16 b | T3B0 | 0.156 ± 0.006 b | 98.6 ± 0.0 e | −1.33 ± 0.02 e | 5.95 ± 0.01 d | ||
| T3L1 | 0.129 ± 0.005 b | 98.5 ± 0.0 c | −2.78 ± 0.02 a | 7.85 ± 0.06 b | 0.64 ± 0.04 | T3B1 | 0.149 ± 0.004 a | 98.3 ± 0.0 e | −1.64 ± 0.07 d | 4.28 ± 0.00 c | 0.72 ± 0.10 | |
| T3L2 | 0.085 ± 0.001 a | 97.9 ± 0.0 b | −1.97 ± 0.03 c | 7.19 ± 0.01 a | 0.28 ± 0.00 | T3B2 | 0.145 ± 0.001 a | 98.4 ± 0.2 e | −1.59 ± 0.01 d | 4.73 ± 0.00 b | 0.66 ± 0.00 | |
| Sixth month | T6L0 | 0.133 ± 0.006 b | 98.7 ± 0.0 c | −1.94 ± 0.03 c | 7.92 ± 0.05 b | T6B0 | 0.162 ± 0.003 c | 98.2 ± 0.0 e | −1.57 ± 0.03 d | 6.74 ± 0.01 e | ||
| T6L1 | 0.189 ± 0.009 c | 98.8 ± 0.0 c | −2.78 ± 0.02 a | 8.86 ± 0.02 c | 1.84 ± 0.07 | T6B1 | 0.158 ± 0.011 b | 97.5 ± 0.0 f | −1.18 ± 0.01 f | 7.35 ± 0.07 f | 1.72 ± 0.11 | |
| T6L2 | 0.110 ± 0.004 d | 98.6 ± 0.1 c | −1.97 ± 0.03 c | 7.15 ± 0.03 a | 0.09 ± 0.00 | T6B2 | 0.155 ± 0.002 b | 89.3 ± 0.0 b | −1.13 ± 0.00 f | 6.27 ± 0.05 e | 8.98 ± 0.01 | |
| Ninth month | T9L0 | 0.166 ± 0.006 e | 98.9 ± 0.1 c | −1.95 ± 0.02 c | 8.81 ± 0.01 c | T9B0 | 0.167 ± 0.002 c | 97.6 ± 0.0 f | −1.14 ± 0.00 f | 7.23 ± 0.02 f | ||
| T9L1 | 0.193 ± 0.003 c | 99.0 ± 0.0 a | −3.07 ± 0.04 d | 8.89 ± 0.02 c | 1.19 ± 0.09 | T9B1 | 0.180 ± 0.003 d | 97.4 ± 0.0 f | −1.17 ± 0.00 f | 9.40 ± 0.24 g | 0.70 ± 0.03 | |
| T9L2 | 0.157 ± 0.001 e | 98.9 ± 0.0 c | −2.07 ± 0.05 c | 7.16 ± 0.03 a | 0.60 ± 0.28 | T9B2 | 0.156 ± 0.005 b | 89.2 ± 0.0 b | −1.52 ± 0.07 d | 6.55 ± 0.04 e | 8.38 ± 0.02 | |
| Wine | Pseudo Zero-Order | k | r | p-Value < |
|---|---|---|---|---|
| Loureiro (L) | Stage 0 | 0.220 ± 0.020 a | 0.968 | 0.0001 |
| Stage 1 | 0.253 ± 0.030 a | 0.949 | 0.0001 | |
| Stage 2 | 0.0122 ± 0.0278 | 0.247 | 0.689 | |
| Blend (B) | Stage 0 | 0.409 ± 0.045 a | 0.956 | 0.0001 |
| Stage 1 | 0.616 ± 0.068 | 0.955 | 0.0001 | |
| Stage 2 | 0.256 ± 0.058 a | 0.931 | 0.0217 |
| Total Phenols (mg/L Gallic Acid) | Non-Flavonoid Phenols (mg/L Gallic Acid) | Flavonoid Phenols (mg/L Gallic Acid) | Gelatin Index (%) | Total Tannins (g/L) | Total Phenols (mg/L Gallic Acid) | Non-Flavonoid Phenols (mg/L Gallic Acid) | Flavonoid Phenols (mg/L Gallic Acid) | Gelatin Index (%) | Total Tannins (g/L) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loureiro Monovarietal Wine | Blend Wine | |||||||||||
| Initial Time | T0L0 | 399 ± 1 a | 148 ± 1 a | 251 ± 1 a | 9 ± 0 a | 0.2 ± 0.0 a | T0B0 | 558 ± 2 a | 226 ± 5 a | 332 ± 3 a | 3 ± 0 a | 0.4 ± 0.0 a |
| T0L1 | 397 ± 4 a | 153 ± 2 a | 244 ± 5 a | 9 ± 1 a | 0.2 ± 0.0 a | T0B1 | 580 ± 6 a | 227 ± 5 a | 343 ± 7 a | 3 ± 0 a | 0.4 ± 0.0 a | |
| T0L2 | 390 ± 1 b | 151 ± 1 a | 239 ± 2 b | 8 ± 1 b | 0.2 ± 0.0 a | T0B2 | 513 ± 6 b | 228 ± 3 a | 285 ± 6 c | 3 ± 0 a | 0.4 ± 0.0 a | |
| First Month | T1L0 | 377 ± 4 b | 147 ± 1 a | 230 ± 3 b | 7 ± 2 c | 0.2 ± 0.0 a | T1B0 | 502 ± 1 c | 181 ± 1 b | 321 ± 1 a | 1 ± 0 c | 0.4 ± 0.0 a |
| T1L1 | 370 ± 4 b | 145 ± 2 a | 225 ± 3 b | 8 ± 1 b | 0.2 ± 0.0 a | T1B1 | 518 ± 4 b | 168 ± 2 b | 350 ± 4 b | 1 ± 0 c | 0.4 ± 0.0 a | |
| T1L2 | 381 ± 4 a | 150 ± 3 a | 231 ± 4 b | 7 ± 0 d | 0.2 ± 0.0 a | T1B2 | 451 ± 1 d | 175 ± 2 b | 276 ± 2 c | 2 ± 0 b | 0.4 ± 0.0 a | |
| Third month | T3L0 | 364 ± 4 c | 144 ± 1 a | 220 ± 4 c | 9 ± 2 a | 0.2 ± 0.0 a | T3B0 | 508 ± 1 c | 177 ± 4 b | 331 ± 4 a | 1 ± 0 c | 0.4 ± 0.0 a |
| T3L1 | 367 ± 4 c | 141 ± 3 a | 226 ± 5 c | 8 ± 0 b | 0.2 ± 0.0 a | T3B1 | 509 ± 6 c | 200 ± 4 c | 309 ± 6 a | 1 ± 0 c | 0.4 ± 0.0 a | |
| T3L2 | 352 ± 2 c | 143 ± 1 a | 209 ± 3 c | 8 ± 0 b | 0.2 ± 0.0 a | T3B2 | 491 ± 5 e | 217 ± 5 d | 274 ± 7 c | 1 ± 0 c | 0.4 ± 0.0 a | |
| Sixth month | T6L0 | 355 ± 2 c | 134 ± 5 b | 221 ± 4 c | 7 ± 0 d | 0.2 ± 0.0 a | T6B0 | 487 ± 2 e | 162 ± 1 e | 325 ± 5 a | 1 ± 0 c | 0.4 ± 0.0 a |
| T6L1 | 360 ± 1 c | 137 ± 1 b | 223 ± 8 c | 8 ± 0 b | 0.2 ± 0.0 a | T6B1 | 474 ± 4 e | 198 ± 5 c | 276 ± 1 c | 1 ± 0 c | 0.4 ± 0.0 a | |
| T6L2 | 352 ± 5 c | 129 ± 4 b | 223 ± 8 c | 6 ± 1 d | 0.2 ± 0.0 a | T6B2 | 479 ± 2 e | 205 ± 1 c | 274 ± 3 c | 1 ± 0 c | 0.4 ± 0.0 a | |
| Ninth month | T9L0 | 321 ± 1 d | 132 ± 4 b | 189 ± 2 d | 6 ± 1 d | 0.2 ± 0.0 a | T9B0 | 441 ± 3 d | 149 ± 3 e | 292 ± 8 c | 1 ± 0 c | 0.4 ± 0.0 a |
| T9L1 | 359 ± 1 c | 132 ± 2 b | 227 ± 9 c | 7 ± 0 d | 0.2 ± 0.0 a | T9B1 | 385 ± 6 f | 126 ± 1 f | 259 ± 8 d | 1 ± 0 c | 0.4 ± 0.0 a | |
| T9L2 | 341 ± 4 c | 127 ± 5 b | 214 ± 5 c | 6 ± 0 d | 0.2 ± 0.0 a | T9B2 | 445 ± 5 d | 186 ± 2 b | 259 ± 3 d | 1 ± 0 c | 0.4 ± 0.0 a | |
| Wine | Pseudo Zero-Order | k | r | p < |
|---|---|---|---|---|
| Loureiro (L) | Stage 0 | 7.61 ± 0.57 a | 0.953 | 0.0001 |
| Stage 1 | 6.52 ± 1.14 a | 0.957 | 0.0001 | |
| Stage 2 | 5.15 ± 1.39 a | 0.906 | 0.0342 | |
| Blend (B) | Stage 0 | 3.79 ± 0.39 | 0.917 | 0.0001 |
| Stage 1 | 6.56 ± 0.71 a | 0.909 | 0.0001 | |
| Stage 2 | 6.38 ± 0.86 a | 0.977 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipe-Ribeiro, L.; Rodrigues, S.; Nunes, F.M.; Cosme, F. Reducing the Negative Effect on White Wine Chromatic Characteristics Due to the Oxygen Exposure during Transportation by the Deoxygenation Process. Foods 2021, 10, 2023. https://doi.org/10.3390/foods10092023
Filipe-Ribeiro L, Rodrigues S, Nunes FM, Cosme F. Reducing the Negative Effect on White Wine Chromatic Characteristics Due to the Oxygen Exposure during Transportation by the Deoxygenation Process. Foods. 2021; 10(9):2023. https://doi.org/10.3390/foods10092023
Chicago/Turabian StyleFilipe-Ribeiro, Luís, Susete Rodrigues, Fernando M. Nunes, and Fernanda Cosme. 2021. "Reducing the Negative Effect on White Wine Chromatic Characteristics Due to the Oxygen Exposure during Transportation by the Deoxygenation Process" Foods 10, no. 9: 2023. https://doi.org/10.3390/foods10092023