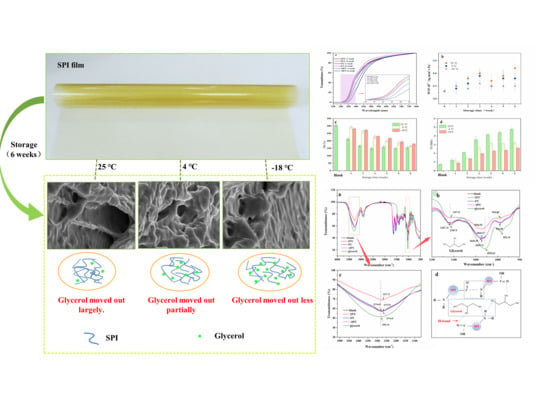
Changes in Properties of Soy Protein Isolate Edible Films Stored at Different Temperatures: Studies on Water and Glycerol Migration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SPI Films
2.3. Storage Conditions
2.4. Thickness
2.5. Swelling Ratio
2.6. Glycerol Migration Rate
2.7. Water Content and Distribution
2.8. ATR-FTIR Analysis
2.9. Thermogravimetric Analysis (TGA)
2.10. Differential Scanning Calorimetry (DSC)
2.11. Transmittance of the Films
2.12. SEM
2.13. Water Vapor Permeability (WVP)
2.14. Mechanical Properties
2.15. Statistical Analysis
3. Results and Discussion
3.1. Thickness
3.2. Migration and Distribution of Glycerol
3.2.1. Glycerol Migration Rate
3.2.2. Differential Scanning Calorimetry (DSC)
3.3. Migration and Distribution of Water
3.3.1. Water Content and Distribution
3.3.2. Thermogravimetric Analysis (TGA)
3.4. Microcosmic State
3.4.1. SEM
3.4.2. ATR-FTIR
3.5. Physical Properties of SPI Films
3.5.1. Swelling Ratio
3.5.2. Transmittance of Films
3.5.3. Water Vapor Permeability (WVP)
3.5.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piccirilli, G.N.; Soazo, M.; Perez, L.M.; Delorenzi, N.J.; Verdini, R.A. Effect of storage conditions on the physicochemical characteristics of edible films based on whey protein concentrate and liquid smoke—ScienceDirect. Food Hydrocoll. 2019, 87, 221–228. [Google Scholar] [CrossRef]
- Kokoszka, S.; Debeaufort, F.; Hambleton, A.; Lenart, A.; Voilley, A. Protein and glycerol contents affect physico-chemical properties of soy protein isolate-based edible films. Innov. Food Sci. Emerg. Technol. 2010, 11, 503–510. [Google Scholar] [CrossRef]
- Neira, L.M.; Martucci, J.F.; Stejskal, N.; Ruseckaite, R.A. Time-dependent evolution of properties of fish gelatin edible films enriched with carvacrol during storage. Food Hydrocoll. 2019, 94, 304–310. [Google Scholar] [CrossRef]
- López-Castejón, M.L.; Bengoechea, C.; García-Morales, M.; Martínez, I. Effect of plasticizer and storage conditions on thermomechanical properties of albumen/tragacanth based bioplastics. Food Bioprod. Process. 2014, 95, 264–271. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Storage-induced changes in functional properties of glycerol plasticized—Soybean protein concentrate films produced by casting—ScienceDirect. Food Hydrocoll. 2015, 45, 247–255. [Google Scholar] [CrossRef]
- Anker, M.; Stading, M.; Hermansson, A.M. Aging of whey protein films and the effect on mechanical and barrier properties. Agric. Food Chem. 2001, 49, 989–995. [Google Scholar] [CrossRef]
- Kang, H.; Song, X.; Wang, Z.; Zhang, W.; Zhang, S.; Li, J. High-Performance and Fully Renewable Soy Protein Isolate-Based Film from Microcrystalline Cellulose via Bio-Inspired Poly(dopamine) Surface Modification. ACS Sustain. Chem. Eng. 2016, 4, 4354–4360. [Google Scholar] [CrossRef]
- Galus, S. Functional properties of soy protein isolate edible films as affected by rapeseed oil concentration. Food Hydrocoll. 2018, 85, 233–241. [Google Scholar] [CrossRef]
- Liu, P.; Xu, H.; Zhao, Y.; Yang, Y. Rheological properties of soy protein isolate solution for fibers and films. Food Hydrocoll. 2017, 64, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Maryam Adilah, Z.A.; Nur Hanani, Z.A. Storage stability of soy protein isolate films incorporated with mango kernel extract at different temperature. Food Hydrocoll. 2018, 87, 541–549. [Google Scholar] [CrossRef]
- Koupantsis, T.; Pavlidou, E.; Paraskevopoulou, A. Glycerol and tannic acid as applied in the preparation of milk proteins—CMC complex coavervates for flavour encapsulation. Food Hydrocoll. 2016, 57, 62–71. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Gu, W.; Liu, X.; Li, F.; Shi, S.Q.; Xia, C.; Zhou, W.; Zhang, D.; Gong, S.; Li, J. Tough, strong, and biodegradable composite film with excellent UV barrier performance comprising soy protein isolate, hyperbranched polyester, and cardanol derivative. Green Chem. 2019, 21, 3651–3665. [Google Scholar] [CrossRef]
- Ket-On, A.; Pongmongkol, N.; Somwangthanaroj, A.; Janjarasskul, T.; Tananuwong, K. Properties and storage stability of whey protein edible film with spice powders. J. Food Sci. Technol. 2016, 53, 2933–2942. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Li, Q.; Zhang, P. Soy Protein Isolate and Glycerol Hydrogen Bonding Using Two-Dimensional Correlation (2D-COS) Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopy. Appl. Spectrosc. 2017, 71, 2437–2445. [Google Scholar] [CrossRef]
- Bagheri, F.; Radi, M.; Amiri, S. Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocoll. 2018, 90, 162–171. [Google Scholar] [CrossRef]
- Tian, H.F.; Guo, G.P.; Xiang, A.M.; Zhong, W.H. Intermolecular interactions and microstructure of glycerol-plasticized soy protein materials at molecular and nanometer levels. Polym. Test. 2018, 67, 197–204. [Google Scholar] [CrossRef]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Álvarez Igarzabal, C.I. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll. 2018, 89, 758–764. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Xu, L.N.; Yan, W.B.; Zhang, H.J.; Chi, Y.J.; Liu, Y.Y.; Wu, Y.Q.; Yin, T.T. Impact of Glycerol-Based Biopolyester on Mechanical Stability of Soybean Protein Composite Films during Storage. Food Sci. Chin. 2018, 39, 207–213. [Google Scholar]
- Lv, C.; Tian, H.; Zhang, X.; Xiang, A. LF-NMR analysis of the water mobility, state and distribution in sorbitol plasticized polyvinyl alcohol films—ScienceDirect. Polym. Test. 2018, 70, 67–72. [Google Scholar] [CrossRef]
- Kevij, H.T.; Salami, M.; Mohammadian, M.; Khodadadi, M. Fabrication and investigation of physicochemical, food simulant release, and antioxidant properties of whey protein isolate-based films activated by loading with curcumin through the pH-driven method. Food Hydrocoll. 2020, 108, 106026. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Wang, K.; Xu, H. Development and evaluation of soy protein isolate-based antibacterial nanocomposite films containing cellulose nanocrystals and zinc oxide nanoparticles. Food Hydrocoll. 2020, 106, 105898. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Wang, Q.; Wu, G.; Liu, C.; Jiang, B.; Xu, J. Effect of Antioxidant and Antimicrobial Coating based on Whey Protein Nanofibrils with TiO2 Nanotubes on the Quality and Shelf Life of Chilled Meat. Int. J. Mol. Sci. 2019, 20, 1184. [Google Scholar] [CrossRef] [Green Version]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocoll. 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Perez, L.M.; Piccirilli, G.N.; Delorenzi, N.J.; Verdini, R.A. Effect of different combinations of glycerol and/or trehalose on physical and structural properties of whey protein concentrate-based edible films. Food Hydrocoll. 2016, 56, 352–359. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Castillo, L.A.; Barbosa, S.E.; De Angelis, M.G. Barrier properties and mechanical strength of bio-renewable, heat-sealable films based on gelatin, glycerol and soybean oil for sustainable food packaging. React. Funct. Polym. 2018, 125, 29–36. [Google Scholar] [CrossRef]
- Park, H.J.; Bunn, J.M.; Weller, C.L.; Vergano, P.J.; Testin, R.F. Water Vapor Permeability and Mechanical Properties of Grain Protein-Based Films as Effected by Mixtures of Polyethylene Glycol amd Glycerin Plasticizers. Trans. ASAE 1994, 37, 1281. [Google Scholar] [CrossRef]
- Tang, C.H.; Xiao, M.L.; Chen, Z.; Yang, X.Q.; Yin, S.W. Properties of cast films of vicilin-rich protein isolates from Phaseolus legumes: Influence of heat curing. LWT Food Sci. Technol. 2009, 42, 1659–1666. [Google Scholar] [CrossRef]
- Tang, C.H.; Choi, S.M.; Ma, C.Y. Study of thermal properties and heat-induced denaturation and aggregation of soy proteins by modulated differential scanning calorimetry. Int. J. Biol. Macromol. 2007, 40, 96–104. [Google Scholar] [CrossRef]
- Perotto, G.; Simonutti, R.; Ceseracciu, L.; Mauri, M.; Athanassiou, A. Water-induced plasticization in vegetable-based bioplastic films: A structural and thermo-mechanical study. Polymer 2020, 200, 122598. [Google Scholar] [CrossRef]
- Liang, Y.; Qu, Z.; Liu, M.; Zhu, M.; Wang, J. Further interpretation of the strengthening effect of curdlan on frozen cooked noodles quality during frozen storage: Studies on water state and properties. Food Chem. 2020, 346, 128908. [Google Scholar] [CrossRef]
- Benet, J.C.; Ouoba, S.; Ouedraogo, F.; Cherblanc, F. Experimental study of water evaporation rate, at the surface of aqueous solution, under the effect of a discontinuity of chemical potential—Effect of water activity and air pressure—ScienceDirect. Exp. Therm. Fluid Sci. 2020, 121, 110233. [Google Scholar] [CrossRef]
- Gao, C.; Pollet, E.; Averous, L. Properties of glycerol-plasticized alginate films obtained by thermo-mechanical mixing. Food Hydrocoll. 2017, 63, 414–420. [Google Scholar] [CrossRef]
- Anker, M.; Stading, M.; Hermansson, A.M. Relationship between the microstructure and the mechanical and barrier properties of whey protein films. J. Agric. Food Chem. 2000, 48, 3806–3816. [Google Scholar] [CrossRef]
- Olabarrieta, I.; Cho, S.W.; Gallstedt, M.; Sarasu, J.R.; Johansson, E.; Hedenqvist, M.S. Aging properties of films of plasticized vital wheat gluten cast from acidic and basic solutions. Biomacromolecules 2006, 7, 1657–1664. [Google Scholar] [CrossRef]
- Li, T.; Xia, N.; Xu, L.; Zhang, H.; Li, H. Preparation, characterization and application of SPI-based blend film with antioxidant activity. Food Packag. Shelf Life 2021, 27, 100614. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef] [Green Version]
- García, A.; Pérez, L.M.; Piccirilli, G.N.; Verdini, R.A. Evaluation of antioxidant, antibacterial and physicochemical properties of whey protein-based edible films incorporated with different soy sauces. LWT 2020, 117, 108587. [Google Scholar] [CrossRef]
- Kchaou, H.; Jridi, M.; Benbettaieb, N.; Debeaufort, F.; Nasri, M. Bioactive films based on cuttlefish (Sepia officinalis) skin gelatin incorporated with cuttlefish protein hydrolysates: Physicochemical characterization and antioxidant properties. Food Packag. Shelf Life 2020, 24, 100477. [Google Scholar] [CrossRef]
- Leerahawong, A.; Tanaka, M.; Okazaki, E.; Osako, K. Stability of the Physical Properties of Plasticized Edible Films from Squid (Todarodes pacificus) Mantle Muscle during Storage. J. Food Sci. 2012, 77, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Artharn, A.; Prodpran, T.; Benjakul, S. Round scad protein-based film: Storage stability and its effectiveness for shelf-life extension of dried fish powder. LWT Food Sci. Technol. 2009, 42, 1238–1244. [Google Scholar] [CrossRef]
- Borneo, R.; Alba, N.; Aguirre, A. New films based on triticale flour: Properties and effects of storage time. J. Cereal Sci. 2016, 68, 82–87. [Google Scholar] [CrossRef]
- Leceta, I.; Penalba, M.; Arana, P.; Guerrero, P.; De La Caba, K. Ageing of chitosan films: Effect of storage time on structure and optical, barrier and mechanical properties. Eur. Polym. J. 2015, 66, 170–179. [Google Scholar] [CrossRef]






| Thickness | |||||||
|---|---|---|---|---|---|---|---|
| Storage Time (Weeks) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Control 25 °C | 0.094 ± 0.002 a | 0.094 ± 0.001 a | 0.094 ± 0.003 a | 0.093 ± 0.000 a | 0.092 ± 0.001 ab | 0.089 ± 0.001 b | 0.088 ± 0.001 b |
| Control 4 °C | 0.094 ± 0.001 a | 0.095 ± 0.003 a | 0.093 ± 0.002 a | 0.094 ± 0.002 a | 0.093 ± 0.004 a | 0.091 ± 0.002 a | 0.093 ± 0.003 a |
| Control −18 °C | 0.094 ± 0.002 a | 0.094 ± 0.004 a | 0.095 ± 0.02 a | 0.093 ± 0.003 a | 0.092 ± 0.002 a | 0.093 ± 0.001 a | 0.094 ± 0.002 a |
| Temperature | Storage Time (Weeks) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| 25 °C | 16.37 ± 0.15 a | 16 ± 0.17 a | 15.43 ± 0.15 b | 14.97 ± 0.12 c | 14.27 ± 0.15 d | 13.43 ± 0.21 e | 12.33 ± 0.21 f |
| 4 °C | 16.37 ± 0.15 a | 16.33 ± 0.15 a | 15.9 ± 0.17 b | 14.90 ± 0.10 c | 14.53 ± 0.15 d | 13.73 ± 0.15 e | 13.47 ± 0.15 e |
| −18 °C | 16.37 ± 0.15 a | 16.20 ± 0.10 ab | 15.97 ± 0.21 b | 15.63 ± 0.12 c | 15.27 ± 0.15 d | 14.73 ± 0.12 e | 14.57 ± 0.15 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, L.; Li, H.; Chi, Y.; Zhang, H.; Xia, N.; Ma, Y.; Jiang, L.; Zhang, X. Changes in Properties of Soy Protein Isolate Edible Films Stored at Different Temperatures: Studies on Water and Glycerol Migration. Foods 2021, 10, 1797. https://doi.org/10.3390/foods10081797
Zhang H, Wang L, Li H, Chi Y, Zhang H, Xia N, Ma Y, Jiang L, Zhang X. Changes in Properties of Soy Protein Isolate Edible Films Stored at Different Temperatures: Studies on Water and Glycerol Migration. Foods. 2021; 10(8):1797. https://doi.org/10.3390/foods10081797
Chicago/Turabian StyleZhang, Hong, Lechuan Wang, Hanyu Li, Yujie Chi, Huajiang Zhang, Ning Xia, Yanqiu Ma, Longwei Jiang, and Xiaonan Zhang. 2021. "Changes in Properties of Soy Protein Isolate Edible Films Stored at Different Temperatures: Studies on Water and Glycerol Migration" Foods 10, no. 8: 1797. https://doi.org/10.3390/foods10081797