Rapid Screening of 350 Pesticide Residues in Vegetable and Fruit Juices by Multi-Plug Filtration Cleanup Method Combined with Gas Chromatography-Electrostatic Field Orbitrap High Resolution Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Stock Solutions and Standards
2.3. GC-Orbitrap Analytical Conditions
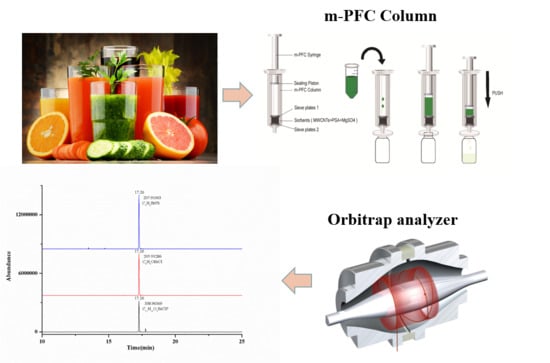
2.4. Sample Preparation
2.5. Database for Screening, Qualitative and Quantitative Rules
3. Results
3.1. Optimization and Comparison of the Extraction Procedure
3.2. Optimization and Comparison of the Cleanup Procedure
3.3. Optimization of Instrument Resolution
3.4. Qualitative Screening and Confirmation
3.5. Matrix Effect
3.6. Validation of the Method
3.7. Practical Screening
3.8. Retrospective Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cortes, A.; Sanchez, M.; Arrebo, L. Fast screening of pesticide residues in fruit juice by solid-phase microextraction and gas chromatography-mass spectrometry. Food Chem. 2008, 107, 1314–1325. [Google Scholar]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A review of the global pesticide legislation and the scale of challenge in reaching the global harmonisation of food safety standards. Integr. Environ. Asses. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Khorram, P.; Nabil, A.A.A. Development of a green liquid-liquid microextraction method using a solid disperser performed in a narrow-bore tube for trace analysis of some organophosphorus pesticides in fruit juices. J. Food Compos. Anal. 2015, 43, 96–105. [Google Scholar] [CrossRef]
- Timofeeva, I.; Shishov, A.; Kanashina, D.; Dzema, D.; Bulatov, A. On-line in-syringe sugaring-out liquid-liquid extraction coupled with HPLC-MS/MS for the determination of pesticides in fruit and berry juices. Talanta 2017, 167, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Suthasinee, B.; Wittaya, N.; Supalax, S. Determination of six pyrethroid insecticides in fruit juice samples using dispersive liquid-liquid microextraction combined with high performance liquid chromatography. Talanta 2012, 88, 209–215. [Google Scholar]
- Bandforuzi, S.R.; Hadjmohammadi, M.R. Modified magnetic chitosan nanoparticles based on mixed hemimicelle of sodium dodecyl sulfate for enhanced removal and trace determination of three organophosphorus pesticides from natural waters. Anal. Chim. Acta 2019, 1078, 90–100. [Google Scholar] [CrossRef]
- Gil García, M.D.; Uclés Duque, S.; Lozano Fernández, A.B.; Sosa, A.; Fernández-Alba, A.R. Multiresidue method for trace pesticide analysis in honeybee wax comb by GC-QqQ-MS. Talanta 2017, 163, 54–64. [Google Scholar] [CrossRef]
- Wang, S.; Qi, P.; Di, S.; Wang, J.; Wu, S.; Wang, X.; Wang, Z.; Wang, Q.; Wang, X.; Zhao, C.; et al. Significant role of supercritical fluid chromatography-mass spectrometry in improving the matrix effect and analytical efficiency during multi-pesticides residue analysis of complex chrysanthemum samples. Anal. Chim. Acta 2019, 1074, 108–116. [Google Scholar] [CrossRef]
- Madej, K.; Kalenik, T.K.; Piekoszewski, W. Sample preparation and determination of pesticides in fat-containing foods. Food Chem. 2018, 269, 527–541. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Bresin, B.; Piol, M.; Fabbro, D.; Mancini, M.A.; Casetta, B.; Del Bianco, C. Analysis of organo-chlorine pesticides residue in raw coffee with a modified “quick easy cheap effective rugged and safe” extraction/clean up procedure for reducing the impact of caffeine on the gas chromatography-mass spectrometry measurement. J. Chromatogr. A 2015, 1376, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, C. Screening of Multi-Pesticide Residues in Edible Fungi by SPE and GC-MS. Food Sci. 2015, 36, 171–175. [Google Scholar]
- Shamsipur, M.; Yazdanfar, N.; Ghambarain, M. Combination of solid-phase extraction with dispersive liquid-liquid microex-traction followed by GC-MS for determination of pesticide residues from water, milk, honey and fruit juice. Food Chem. 2016, 204, 289–297. [Google Scholar] [CrossRef]
- Abdulra’uf, L.B.; Tan, G.H. Chemometric approach to the optimization of HS-SPME/GC-MS for the determination of multiclass pesticide residues in fruits and vegetables. Food Chem. 2015, 177, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xu, X.; Luo, J.; Jin, S.; Chen, W.; Liu, Z.; Tian, C. Simultaneous determination of 131 pesticides in tea by on-line GPC-GC-MS/MS using graphitized multi-walled carbon nanotubes as dispersive solid phase extraction sorbent. Food Chem. 2018, 276, 202–208. [Google Scholar] [CrossRef]
- Luo, Y.B.; Zheng, H.B.; Jiang, X.Y.; Li, X.; Zhang, H.F.; Zhu, F.P.; Pang, Y.Q.; Feng, Y.Q. Determination of Pesticide Residues in Tobacco Using Modified QuEChERS Procedure Coupled to Online Gel Permeation Chromatography-Gas Chromatography/Tandem Mass Spectrometry. Chin. J. Anal. Chem. 2015, 43, 1538–1544. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Evaluation of QuEChERS sample preparation and liquid chromatography-triple-quadrupole mass spectrometry method for the determination of 109 pesticide residues in tomatoes. Food Chem. 2015, 176, 319–332. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, L.; Peng, Y.; Luo, M.; Wang, W.; Liu, X. Multiresidue analysis of over 200 pesticides in cereals using a QuEChERS and gas chromatography-tandem mass spectrometry-based method. Food Chem. 2015, 169, 372–380. [Google Scholar] [CrossRef]
- Huang, Y.S.; Shi, T.; Luo, X.; Xiong, H.L.; Min, F.F.; Chen, Y.; Nie, S.P.; Xie, M.Y. Determination of multi-pesticide residues in green tea with a modified QuEChERS protocol coupled to HPLC-MS/MS. Food Chem. 2019, 275, 255–264. [Google Scholar] [CrossRef]
- Lehotay, S.J. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: Collaborative study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef] [Green Version]
- Foods of Plant Origin-Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE-QuEChERS-Method. Available online: www.cen.eu (accessed on 20 February 2018).
- Zhan, X.P.; Ma, L.; Huang, L.Q.; Chen, J.B.; Zhao, L. The optimization and establishment of QuEChERS-UPLC-MS/MS method for simultaneously detecting various kinds of pesticides residues in fruits and vegetables. J. Chromatogr. B 2017, 1060, 281–290. [Google Scholar]
- Han, Y.; Zou, N.; Song, L.; Li, Y.; Qin, Y.; Liu, S.; Li, X.; Pan, C. Simultaneous determination of 70 pesticide residues in leek, leaf lettuce and garland chrysanthemum using modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials. J. Chromatogr. B 2015, 1005, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tong, M.; Tang, J.; Bian, H.; Wan, X.; He, L.; Hou, R. Analysis of multiple pesticide residues in polyphenol-rich agricultural products by UPLC-MS/MS using a modified QuEChERS extraction and dilution method. Food Chem. 2019, 274, 452–459. [Google Scholar] [CrossRef]
- Chen, L.; Song, F.; Liu, Z.; Zheng, Z.; Xing, J.; Liu, S. Multi-residue method for fast determination of pesticide residues in plants used in traditional chinese medicine by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2012, 1225, 132–140. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, J.; Zhang, Y.; Li, F.; Han, Y.; Zou, N.; Xu, H.; Qian, M.; Pan, C. Automated multi-plug filtration cleanup for liquid chromatographic-tandem mass spectrometric pesticide multi-residue analysis in representative crop commodities. J. Chromatogr. A 2016, 1462, 19–26. [Google Scholar] [CrossRef]
- Wu, Y.; An, Q.; Wu, J.; Li, P.; He, J.; Pan, C. Development and evaluation of an automated multi-channel multiplug filtration cleanup device for pesticide residue analysis on mulberry leaves and processed tea. RSC Adv. 2020, 10, 2589–2597. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.J.; Huang, Y.X.; Di, P.Y.; Zhao, L.M.; Niu, L.S.; Fan, S.F.; Li, Q.; Zhang, Y. Rapid Screening of 234 Pesticide Residues in Vegetables and Fruits by Multi-plug Filtration Cleanup Method Combined with Gas Chromatography-Quadrupole Time of Flight Mass Spectrometry. Food Sci. 2020, 41, 272–285. [Google Scholar]
- Khan, Z.; Kamble, N.; Bhongale, A.; Girme, M.; Chauhan, B.V.; Banerjee, K. Analysis of pesticide residues in tuber crops using pressurised liquid extractionand gas chromatography-tandem mass spectrometry. Food Chem. 2018, 241, 250–257. [Google Scholar] [CrossRef]
- Gawei, M.; Kiljanek, T.; Niewiadowska, A.; Semeniuk, S.; Goliszek, M.; Burek, O.; Posyniak, A. Determination of neonicotinoids and 199 other pesticide residuesinhoney by liquid and gas chromatography coupled tandem massspectrometry. Food Chem. 2019, 282, 36–47. [Google Scholar]
- Liu, X.Q.; Li, Y.F.; Meng, W.T.; Li, D.X.; Sun, H.; Tong, L.; Sun, G.X. Amulti-residuemethod for simultaneous determinationof 74 pesticides inChinesematerial medica using modified QuEChERS sample preparationprocedureandgas chromatography tandem massspectrometry. J. Chromatogr. B 2016, 1015, 1–12. [Google Scholar] [CrossRef]
- Hua, J.; Fayyaz, A. Development of a method Sin-QuEChERS for the determination of multiple pesticide residues in oilseed samples. J. Qual. Assur. Saf. Crops Foods 2019, 11, 511–516. [Google Scholar] [CrossRef]
- Le, S.; Yongtao, H. Rapid single-step cleanup method for analyzing 47 pesticide residues inpepper, chili peppers and its sauce product by high performance liquid and gas chromatography-tandem mass spectrometry. Food Chem. 2019, 279, 237–245. [Google Scholar]
- Mol, H.G.; Tienstra, M.; Zomer, P. Evaluation of gas chromatography—Electron ionization—Full scan high resolution Orbitrap mass spectrometry for pesticide residue analysis. Anal. Chim. Acta 2016, 935, 161–172. [Google Scholar] [CrossRef]
- Uclés, S.; Uclés, A.; Martínez-Bueno, M.J.; Fernández-Alba, A.R. Shifting theparadigmin gas chromatography mass spectrometrypesticide analysis usinghigh resolution accurate mass spectrometry. J. Chromatogr. A 2017, 1501, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Marc, T.; Hans, G.J.M. Application of Gas Chromatography Coupled to Quadrupole-Orbitrap Mass Spectrometry for Pesticide Residue Analysisin Cereals and Feed Ingredients. J. AOAC Int. 2018, 101, 342–351. [Google Scholar]
- Lozano, A.; Uclés, S.; Uclés, A.; Ferrer, C.; Fernández-Alba, A.R. Pesticide Residue Analysis in Fruit- and Vegetable-Based Baby Foods Using GC-Orbitrap MS. J. AOAC Int. 2018, 101, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed, SANTE/12682/2019; European Commission Directorate General for Health and Food Safety: Brussels, Belgium, 2019.
- China, National Food Safety Standard-Maximum Residue Limits for Pesticides in Food (GB 2763-2019); China Agriculture Press: Beijing, China, 2019.
- Codex Allimentarius Commission. Pesticide Database-Maximum Residue Limits. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticides/en/ (accessed on 19 April 2021).
- European Commission. EU Pesticides Database-Pesticide Residues. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/products/?event=search.pr (accessed on 19 April 2021).





| Method | Recoveries (%) | RSD (%) | LOD (μg/kg) | LOQ (μg/kg) | Number of Pesticides | Cleanup Time Cost per Sample (Min) |
|---|---|---|---|---|---|---|
| m-PFC | 72–122 | <10.8 | 0.3–3.0 | 1.0–10.0 | 350 | <2 |
| previous m-PFC | 70–125 | <15.0 | 1.0–3.0 | 3.0–10.0 | <200 | 2–3 |
| traditional QuEChERS | 60–125 | <15.0 | 1.0–3.0 | 3.0–10.0 | 250 | 5 |
| Samples | Pesticide | Positive Samples | Range of Residues (μg/kg) | |
|---|---|---|---|---|
| N | % | |||
| orange juice | Propiconazole | 8 | 26.7 | 41.2–200.4 |
| Trifloxystrobin | 14 | 46.7 | 20.8–300.7 | |
| Chlorpyrifos | 12 | 40.0 | 10.5–100.9 | |
| Imazalil | 13 | 43.3 | 30.4–425.9 | |
| Pyraclostrobin | 11 | 36.7 | 30.1–224.5 | |
| Malathion | 2 | 6.7 | 30.6–230.4 | |
| Pyrimethanil | 7 | 23.3 | 20.2–100.7 | |
| profenofos | 14 | 46.7 | 40.2–600.4 | |
| apple juice | Difenoconazole | 8 | 26.7 | 21.2–420.4 |
| Chlorpyrifos | 6 | 20.0 | 36.8–300.7 | |
| Flusilazole | 3 | 10.0 | 50.5–80.9 | |
| Tebuconazole | 15 | 50.0 | 10.1–344.1 | |
| Dimethomorph | 7 | 23.3 | 30.8–250.2 | |
| grape juice | Bifenthrin | 4 | 13.3 | 20.1–200.3 |
| Difenoconazole | 6 | 20.0 | 20.2–600.4 | |
| Dimethomorph | 5 | 16.7 | 50.2–520.4 | |
| Propiconazole | 8 | 26.7 | 16.2–312.7 | |
| Pyraclostrobin | 12 | 40.0 | 25.5–400.9 | |
| Pyrimethanil | 7 | 23.3 | 12.4–465.3 | |
| Spirodiclofen | 9 | 30.0 | 10.1–344.1 | |
| Metalaxyl | 16 | 53.3 | 31.8–750.5 | |
| Chlorfenapyr | 13 | 43.3 | 40.1–500.3 | |
| Tebuconazole | 10 | 33.3 | 15.2–400.4 | |
| strawberry juice | Cyhalothrin | 6 | 20.0 | 20.1–100.3 |
| Dichlorvos | 4 | 13.3 | 10.2–62.4 | |
| celery juice | Chlorfenapyr | 16 | 53.3 | 43.2–520.4 |
| Difenoconazole | 12 | 40.0 | 12.2–344.7 | |
| Metalaxyl | 13 | 43.3 | 20.5–450.9 | |
| Prochloraz | 12 | 40.0 | 43.4–805.3 | |
| Propiconazole | 11 | 36.7 | 30.1–305.1 | |
| Tebuconazole | 8 | 26.7 | 31.8–750.5 | |
| Isoprocarb | 3 | 10.0 | 42.1–80.3 | |
| Dimethomorph | 11 | 36.7 | 15.2–520.4 | |
| Pyraclostrobin | 12 | 40.0 | 21.1–430.3 | |
| Oxadixyl | 7 | 23.3 | 18.2–90.4 | |
| Phorate | 4 | 13.3 | 10.2–400.4 | |
| Metalaxyl | 15 | 50.0 | 12.2–343.7 | |
| Pendimethalin | 9 | 30.0 | 21.5–320.6 | |
| carrot juice | Triadimefon | 6 | 20.0 | 12.2–505.3 |
| Phorate | 3 | 10.0 | 16.1–134.3 | |
| cucumber juice | Metalaxyl | 14 | 46.7 | 31.2–850.5 |
| Procymidone | 11 | 36.7 | 20.1–440.3 | |
| Pyrimethanil | 8 | 26.7 | 15.2–330.4 | |
| Chlorpyrifos | 10 | 33.3 | 22.1–426.3 | |
| Dimethoate | 5 | 16.7 | 20.2–560.4 | |
| Fluopyram | 4 | 13.3 | 20.2–800.4 | |
| Omethoate | 6 | 20.0 | 16.2–122.4 | |
| Dimethomorph | 10 | 33.3 | 18.5–423.9 | |
| Endosulfan | 3 | 10.0 | 12.4–225.3 | |
| Chlorfenapyr | 13 | 43.3 | 12.1–444.1 | |
| Pyridaben | 12 | 40.0 | 31.8–651.5 | |
| tomato juice | Chlorfenapyr | 13 | 43.3 | 38.1–623.3 |
| Difenoconazole | 10 | 33.3 | 15.2–442.4 | |
| Pyrimethanil | 9 | 30.0 | 20.1–330.3 | |
| Dimethomorph | 11 | 36.7 | 16.3–422.1 | |
| Procymidone | 12 | 40.0 | 22.4–445.3 | |
| Tebuconazole | 14 | 46.7 | 10.1–384.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Z.; Li, Q.; Cong, J.; Huang, Y.; Wang, D.; Pan, C.; Fan, S.; Zhang, Y. Rapid Screening of 350 Pesticide Residues in Vegetable and Fruit Juices by Multi-Plug Filtration Cleanup Method Combined with Gas Chromatography-Electrostatic Field Orbitrap High Resolution Mass Spectrometry. Foods 2021, 10, 1651. https://doi.org/10.3390/foods10071651
Meng Z, Li Q, Cong J, Huang Y, Wang D, Pan C, Fan S, Zhang Y. Rapid Screening of 350 Pesticide Residues in Vegetable and Fruit Juices by Multi-Plug Filtration Cleanup Method Combined with Gas Chromatography-Electrostatic Field Orbitrap High Resolution Mass Spectrometry. Foods. 2021; 10(7):1651. https://doi.org/10.3390/foods10071651
Chicago/Turabian StyleMeng, Zhijuan, Qiang Li, Jianhan Cong, Yunxia Huang, Dong Wang, Canping Pan, Sufang Fan, and Yan Zhang. 2021. "Rapid Screening of 350 Pesticide Residues in Vegetable and Fruit Juices by Multi-Plug Filtration Cleanup Method Combined with Gas Chromatography-Electrostatic Field Orbitrap High Resolution Mass Spectrometry" Foods 10, no. 7: 1651. https://doi.org/10.3390/foods10071651