Applications of Lactobacillus acidophilus-Fermented Mango Protected Clostridioides difficile Infection and Developed as an Innovative Probiotic Jam
Abstract
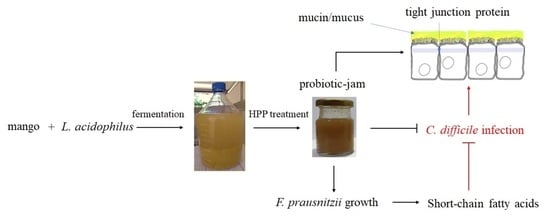
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fermentation
2.3. Cell Culture
2.4. Western Blotting
2.5. L. acidophilus BCRC14079-Fermented Mango Inhibited C. difficile Growth
2.6. C. difficile Infection in Caco-2 Cells
2.7. Investigation of L. acidophilus BCRC14079-Fermented Mango Regulated Growth of F. prausnitzii
2.8. Assay for Short-Chain Fatty Acids (SCFAs)
2.9. Preparation of L. acidophilus BCRC14079-Fermented Mango Jam Treated with High-Pressure Processing (HPP)
2.10. Texture Profile Analysis (TPA)
2.11. Color Measurement
2.12. Sensory Evaluation
2.13. Statistical Analysis
3. Results and Discussion
3.1. Properties of L. acidophilus BCRC14079-Fermented Mango
3.2. Inhibition of the Growth on C. difficile by L. acidophilus BCRC14079-Fermented Mango
3.3. Regulation of L. acidophilus BCRC14079-Fermented Mango on the Growth of F. prausnitzii
3.4. Development of Innovative Probiotics-Fermented Mango Jam
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asempa, T.E.; Nicolau, D.P. Clostridium difficile infection in the elderly: An update on management. Clin. Interv. Aging 2017, 12, 1799. [Google Scholar] [CrossRef] [Green Version]
- Vakili, B.; Fateh, A.; Aghdaei, H.A.; Sotoodehnejadnematalahi, F.; Siadat, S.D. Intestinal microbiota in elderly inpatients with Clostridioides difficile infection. Infect. Drug Resist. 2020, 13, 2723–2731. [Google Scholar] [CrossRef]
- Gu, S.; Chen, Y.; Zhang, X.; Lu, H.; Lv, T.; Shen, P.; Lv, L.; Zheng, B.; Jiang, X.; Li, L. Identification of key taxa that favor intestinal colonization of Clostridium difficile in an adult Chinese population. Microbes Infect. 2016, 18, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Yi, J.; Kim, J.H.; Lee, S.W.; Moon, H.W. Composition of gut microbiota in patients with toxigenic Clostridioides (Clostridium) difficile: Comparison between subgroups according to clinical criteria and toxin gene load. PLoS ONE 2019, 14, e0212626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miquel, S.; Martin, R.; Rossi, O.; Bermudez-Humaran, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.; Stewart, C.S.; Flint, H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. Nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar] [PubMed] [Green Version]
- Khan, M.T.; Duncan, S.H.; Stams, A.J.; van Dili, J.M.; Flint, H.J.; Harmsen, H.J. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J. 2012, 6, 1578–1585. [Google Scholar] [CrossRef]
- Wrzosek, L.; Miquel, S.; Noordine, M.L.; Bouet, S.; Chevalier-Curt, M.J.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Martín, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Bercik, P.; Verdu, E.F.; et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015, 15, 67. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xu, H.; Xu, J.; Guo, X.; Zhao, H.; Chen, Y.; Zhou, Y.; Nie, Y. F. prausnitzii and its supernatant increase SCFAs-producing bacteria to restore gut dysbiosis in TNBS-induced colitis. AMB Express 2021, 11, 33. [Google Scholar] [CrossRef]
- Rabiei, N.; Badi, S.A.; Marvasti, F.E.; Sattari, T.N.; Vaziri, F.; Siadat, S.D. Induction effects of Faecalibacterium prausnitzii and its extracellular vesicles on toll-like receptor signaling pathway gene expression and cytokine level in human intestinal epithelial cells. Cytokine 2019, 121, 154718. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef] [Green Version]
- Reddy, L.; Min, J.H.; Wee, Y.J. Production of probiotic mango juice by fermentation of lactic acid bacteria. Korean J. Microbiol. Biotechnol. 2015, 43, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.Y.; Guo, L.Q.; Ye, Z.W.; Qiu, L.Y.; Gu, F.W.; Lin, J.F. Use of autochthonous lactic acid bacteria starters to ferment mango juice for promoting its probiotic roles. Prep. Biochem. Biotechnol. 2016, 46, 399–405. [Google Scholar] [CrossRef]
- Kamassah, A.K.Q.; Saalia, F.K.; Fosu, P.O.; Mensah-Brown, H.; Sinayobye, E.; Tano-Debrah, K. Fermentation capacity of yeasts using mango (Mangiferia indica Linn.) as substrate. Food. Sci. Qual. Manag. 2013, 22, 69–78. [Google Scholar]
- Reddy, L.V.; Reddy, O.V.S. Production of ethanol from mango (Mangifera indica L.) fruit juice fermentation. Res. J. Microbiol. 2007, 2, 763–769. [Google Scholar]
- Li, X.; Chan, L.J.; Yu, B.; Curran, P.; Liu, S.Q. Fermentation of three varieties of mango juices with a mixture of Saccharomyces cerevisiae and Williopsis saturnus var. mrakii. Int. J. Food Microbiol. 2012, 158, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ordonez-Diaz, J.L.; Moreno-Ortega, A.; Roldan-Guerra, F.J.; Ortiz-Somovilla, V.; Moreno-Rojas, J.M.; Pereira-Caro, G. In vitro gastrointestinal digestion and colonic catabolism of mango (Mangifera indica L.) pulp polyphenols. Foods 2020, 9, 1836. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Maldonado, L.M.; Blancas-Benitez, F.J.; Zamora-Gasga, V.M.; Cardenas-Castro, A.P.; Tovar, J.; Sayago-Ayerdi, S.G. In vitro gastrointestinal digestion and colonic fermentation of high dietary fiber and antioxidant-rich mango (Mangifera indica L.) “Ataulfo”-based fruit bars. Nutrients 2019, 11, 1564. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Sarmiento, W.; Sayago-Ayerdi, S.G.; Goni, I.; Gutierrez-Miceli, F.A.; Abud-Archila, M.; Rejon-Orantes, J.C.; Rincon-Rosales, R.; Pena-Ocana, B.A.; Ruiz-Valdiviezo, V.M. Changes in intestinal microbiota and predicted metabolic pathways during colonic fermentation of mango (Mangifera indica L.)-based bar indigestible fraction. Nutrients 2020, 12, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Klinken, B.J.; Oussoren, E.; Weenink, J.J.; Strous, G.J.; Buller, H.A.; Dekker, J.; Einerhand, A.W. The human intestinal cell lines Caco-2 and LS174T as models to study cell-type specific mucin expression. Glycoconj. J. 1996, 13, 757–768. [Google Scholar] [CrossRef]
- Altamimi, M.; Abdelhay, O.; Rastall, R.A. Effect of oligosaccharides on the adhesion of gut bacteria to human HT-29 cells. Anaerobe 2016, 39, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Chen, Y.W.; Tain, Y.L.; Chang, S.K.C.; Huang, L.T.; Hsieh, C.W.; Hou, C.Y. Fast quantification of short-chain fatty acids in rat plasma by gas chromatography. J. Food Sci. 2020, 85, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Sravani, V.J.; Ravi, N.; Roopa, N.; Kumar, S.; Pandey, A.K.; Chauhan, O.P. Use of high pressure technology for the development of novel jam and its quality evaluation during storage. J. Food Sci. Technol. 2017, 54, 3562–3568. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, S.; Abdul-Malek, S.; Kamdem, R.E.; Jam, K.L. A versatile and inexpensive technique for measuring color of foods. Food Technol. 2000, 54, 48–51. [Google Scholar]
- Hsu, W.H.; Lai, Y.J.; Wu, S.C. Effects of the anti-microbial peptide pardaxin plus sodium erythorbate dissolved in different gels on the quality of Pacific white shrimp under refrigerated storage. Food Control 2017, 73, 712–719. [Google Scholar] [CrossRef]
- Wu, S.C.; Su, Y.S.; Cheng, H.Y. Antioxidant properties of Lactobacillus-fermented and non-fermented Graptopetalum paraguayense E. Walther at different stages of maturity. Food Chem. 2011, 129, 804–809. [Google Scholar] [CrossRef]
- Lee, B.H.; Lo, Y.H.; Pan, T.M. Anti-obesity activity of Lactobacillus fermented soy milk products. J. Funct. Foods 2013, 5, 905–913. [Google Scholar] [CrossRef]
- Tung, Y.T.; Lee, B.H.; Liu, C.F.; Pan, T.M. Optimization of culture condition for ACEI and GABA production by lactic acid bacteria. J. Food Sci. 2011, 76, M585–M591. [Google Scholar] [CrossRef]
- Yang, H.T.; Chen, J.W.; Rathod, J.; Jiang, Y.Z.; Tsai, P.J.; Hung, Y.P.; Ko, W.C.; Paredes-Sabja, D.; Huang, I.H. Lauric acid is an inhibitor of Clostridium difficile growth in vitro and reduces inflammation in a mouse infection model. Front. Microbiol. 2018, 8, 2635. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N. Clostridium difficile infection: Epidemiology, risk factors and management. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 17–26. [Google Scholar] [CrossRef]
- Johnson, S. Recurrent Clostridium difficile infection: A review of risk factors, treatments, and outcomes. J. Infect. 2009, 58, 403–410. [Google Scholar] [CrossRef]
- Johnson, S.; Maziade, P.J.; McFarland, L.V.; Trick, W.; Donskey, C.; Currie, B.; Low, D.E.; Goldstein, E.J.C. Is primary prevention of Clostridium difficile infection possible with specific probiotics? Int. J. Infect. Dis. 2012, 16, e786–e792. [Google Scholar] [CrossRef] [Green Version]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium difficile colitis: Pathogenesis and host defense. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef]
- Spinler, J.; Auchtung, J.; Brown, A.; Boonma, P.; Oezguen, N.; Ross, C.L.; Luna, R.A.; Runge, J.; Versalovic, J.; Peniche, A.; et al. Next-gerneration probiotics targeting Clostridium difficile through precursor-directed antimicrobial biosynthesis. Infect. Immun. 2017, 85, e00303-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahida, Y.R.; Makh, S.; Hyde, S.; Gray, T.; Borriello, S.P. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: Induction of interleukin 8 production and apoptosis after cell detachment. Gut 1996, 38, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Chumbler, N.M.; Farrow, M.A.; Lapierre, L.A.; Franklin, J.L.; Haslam, D.; Goldenring, J.R.; Lacy, D.B. Clostridium difficile toxin B causes epithelial cell necrosis through an autoprocessing-independent mechanism. PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef]
- Arbizu, S.; Chew, B.; Mertens-Talcott, S.U.; Noratto, G. Commercial whey products promote intestinal barrier function with glycomaropeptide enhanced activity in downregulating bacterial endotoxin lipopolysaccharides (LPS)-induced inflammation in vitro. Food Funct. 2020, 11, 5842–5852. [Google Scholar] [CrossRef] [PubMed]
- Gigli, S.; Seguella, L.; Pesce, M.; Bruzzese, E.; D’Alessandro, A.; Cuomo, R.; Steardo, L.; Sarnelli, G.; Esposito, G. Cannabidiol restores intestinal barrier dysfunction and inhibits the apoptotic process induced by Clostridium difficile toxin A in Caco-2 cells. United Eur. Gastroenterol. J. 2017, 5, 1108–1115. [Google Scholar] [CrossRef] [Green Version]
- Janvilisri, T.; Scaria, J.; Chang, Y.F. Transcriptional profiling of Clostridium difficile and Caco-2 cells during infection. J. Infect. Dis. 2010, 202, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pothoulakis, C. Effects of Clostridium difficile toxins on epithelial cell barrier. Ann. N. Y. Acad. Sci. 2000, 915, 347–356. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Verhoog, S.; Taneri, P.E.; Roa Diaz, Z.M.; Marques-Vidal, P.; Troup, J.P.; Bally, L.; Franco, O.H.; Glisic, M.; Muka, T. Dietary factors and modulation of bacteria strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A systematic review. Nutrients 2019, 11, 1565. [Google Scholar] [CrossRef] [Green Version]
- Heinken, A.; Khan, M.T.; Paglia, G.; Rodionov, D.A.; Harmsen, H.J.; Thiele, I. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J. Bacteriol. 2014, 196, 3289–3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roychowdhury, S.; Cadnum, J.; Glueck, B.; Obrenovich, M.; Donskey, C.; Cresci, G.A.M. Faecalibacterium prausnitzii and a prebiotic protect intestinal health in a mouse model of antibiotic and Clostridium difficile exposure. JPEN J. Parenter. Enteral Nutr. 2018, 42, 1156–1167. [Google Scholar] [CrossRef]
- Gaisawat, M.B.; Iskandar, M.M.; MacPherson, C.W.; Tompkins, T.A.; Kubow, S. Probiotic supplementation is associated with increased antioxidant capability and copper chelation in C. difficile-infected fecal water. Nutrients 2019, 11, 2007. [Google Scholar] [CrossRef] [Green Version]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Grtadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microb. 2015, 17, 565–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyles, L.; Snelling, T.; Umlai, U.K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-host systems interactions: Protective effects of propionate upon the blood-brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Nourmohammadi, A.; Ahmadi, E.; Heshmati, A. Optimization of physicochemical, textural, and rheological properties of sour cherry jam containing stevioside by using response surface methodology. Food Sci. Nutr. 2021, 9, 2483–2496. [Google Scholar] [CrossRef]
- Dordevic, D.; Jancikova, S.; Capikova, J.; Tremlová, B.; Kushkevych, I. Chemical and sensory properties of fruit jams affected by bamboo fiber fortification. Biointerface Res. Appl. Chem. 2020, 10, 5247–5251. [Google Scholar]
- Mousavi, M.; Heshmati, A.; Garmakhany, A.D.; Vahidinia, A.; Taheri, M. Optimization of the viability of Lactobacillus acidophilus and physico-chemical, textural and sensorial characteristics of flaxseed enriched stirred probiotic yogurt by using response surface methodology. LWT Food Sci. Technol. 2019, 102, 80–88. [Google Scholar] [CrossRef]
- Garrido, J.I.; Lozano, J.E.; Genovese, D.B. Effect of formulation variables on rheology, texture, colour, and acceptability of apple jelly: Modelling and optimization. LWT Food Sci. Technol. 2015, 62, 325–332. [Google Scholar] [CrossRef]
- Azari-Anpar, M.; Payeinmahali, H.; Daraei Garmakhany, A.; Sadeghi Mahounak, A. Physicochemical, microbial, antioxidant, and sensory properties of probiotic stirred yoghurt enriched with Aloe vera foliar gel. J. Food Proc. Preserv. 2017, 41, e13209. [Google Scholar] [CrossRef]
- Martin, I.; Rodriguez, A.; Sanchez-Montero, L.; Padilla, P.; Cordoba, J.J. Effect of the dry-cured fermented sausage “Salchichon” processing with a selected Lactobacillus sakei in Listeria monocytogenes and microbial population. Foods 2021, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Mashitoa, F.M.; Akinola, S.A.; Manhevi, V.E.; Garcia, C.; Remize, F.; Slabbert, R.M.; Sivakumar, D. Influence of fermentation of pasteurized papaya puree with different lactic acid bacterial strains on quality and bioaccessibility of phenolic compounds during in vitro digestion. Foods 2021, 10, 962. [Google Scholar] [CrossRef] [PubMed]







| Short-Chain Fatty Acids | Acetic Acid | Propionic Acid | Butyric Acid |
|---|---|---|---|
| Concentration (μM) | |||
| Control | 103.0 ± 13.2 c | 9.5 ± 1.5 | 1477.0 ± 210.6 b |
| Mango extracts | 168.1 ± 18.2 b | 11.3 ± 3.1 | 1892.0 ± 141.7 b |
| Mango-fermented supernatant extracts | 228.3 ± 12.5 a | 10.6 ± 2.6 | 2521.0 ± 115.3 a |
| Mango-fermented precipitate extracts | 241.5 ± 10.6 a | 12.8 ± 2.2 | 2263.0 ± 179.1 a |
| Groups | HPP Treatment (MPa) | Heat Treatment | ||
|---|---|---|---|---|
| 150 | 300 | 500 | ||
| L. acidophilus BCRC14079-fermented mango solution (%) | 53.5 | 53.5 | 53.5 | 53.5 |
| pH | 3.05 | 3.05 | 3.05 | 3.05 |
| Total sugars (%) | 45 | 45 | 45 | 45 |
| Pectin (%) | 1.5 | 1.5 | 1.5 | 1.5 |
| L* | 30.76 | 30.53 | 31.81 | 35.49 |
| a* | −3.16 | −3.16 | −3.14 | −2.66 |
| b* | 8.14 | 8.53 | 8.37 | 11.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-H.; Hsu, W.-H.; Chien, H.-Y.; Hou, C.-Y.; Hsu, Y.-T.; Chen, Y.-Z.; Wu, S.-C. Applications of Lactobacillus acidophilus-Fermented Mango Protected Clostridioides difficile Infection and Developed as an Innovative Probiotic Jam. Foods 2021, 10, 1631. https://doi.org/10.3390/foods10071631
Lee B-H, Hsu W-H, Chien H-Y, Hou C-Y, Hsu Y-T, Chen Y-Z, Wu S-C. Applications of Lactobacillus acidophilus-Fermented Mango Protected Clostridioides difficile Infection and Developed as an Innovative Probiotic Jam. Foods. 2021; 10(7):1631. https://doi.org/10.3390/foods10071631
Chicago/Turabian StyleLee, Bao-Hong, Wei-Hsuan Hsu, Hao-Yuan Chien, Chih-Yao Hou, Ya-Ting Hsu, You-Zuo Chen, and She-Ching Wu. 2021. "Applications of Lactobacillus acidophilus-Fermented Mango Protected Clostridioides difficile Infection and Developed as an Innovative Probiotic Jam" Foods 10, no. 7: 1631. https://doi.org/10.3390/foods10071631