Extending the Shelf-Life of Fresh-Cut Green Bean Pods by Ethanol, Ascorbic Acid, and Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
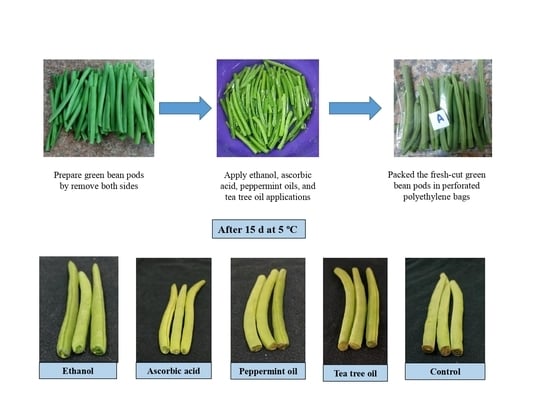
2.1. Preparation of Plant Material and Treatments
- A.
- Ethanol (0.5%),
- B.
- Ascorbic acid (AsA) (0.5%),
- C.
- Tea tree oil (TTO) (0.5%), and
- D.
- Peppermint oil (PMO) (0.5%).
2.2. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
2.3. Free Radical Scavenging Using DPPH
2.4. Determination of Weight Loss, General Appearance, Firmness, and Total Soluble Solids
2.5. Determination of Total Sugar, Vitamin C, Total Phenolic Compounds, and Chlorophyll Content
2.6. Determination of Mould, Yeast and Total Microbial Count
2.7. Statistical Analysis
3. Results
3.1. Chemical Composition of Essential Oils and Free Radical Scavenging
3.2. Appearance
3.3. Weight Loss
3.4. Firmness
3.5. Chlorophyll Content
3.6. Vitamin C
3.7. Total Soluble Solids (TSS)
3.8. Total Phenolic Compounds (TPC)
3.9. Total Sugars
3.10. Mould, Yeast and Total Microbial Count
4. Discussion
4.1. Chemical Composition of Essential Oils and Free Radical Scavenging
4.2. Appearance
4.3. Weight Loss
4.4. Firmness
4.5. Chlorophyll Content
4.6. Vitamin C
4.7. Total Soluble Solids (TSS)
4.8. Total Phenolic Compounds (TPC)
4.9. Total Sugars
4.10. Mould & Yeast (MY) and Total Microbial Count
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brigide, P.; Canniatt-Brazaca, S.G.; Silva, M.O. Nutritional characteristics of biofortified common beans. Food Sci. Technol. 2014, 34, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.F.; Zhang, M.S. Research advances in the postharvest storage and preservation techniques of fresh common bean (Phaseolus vulgaris L.). Sci. Technol. Food Ind. 2019, 40, 326–330. [Google Scholar]
- Miao, Y.; Tian, W.N.; Hao, C.M.; Rao, L.; Cao, J.K.; Jiang, W.B. Study on pods fibrosis delaying of postharvest common bean by chitosan treatment. J. China Agr. Univ. 2012, 17, 132–137. [Google Scholar]
- El-Mogy, M.M.; Alsanius, B.W. Cassia oil for controlling plant and human pathogens on fresh strawberries. Food Control 2012, 28, 157–162. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Idaomar, M. Biological effects of essential oils–A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, J.; Wei, Y.; Han, P.; Dai, K.; Zou, X.; Jiang, S.; Xu, F.; Wang, H.; Sun, J.; et al. Tea tree oil controls brown rot in peaches by damaging the cell membrane of Monilinia fructicola. Postharvest Biol. Technol. 2021, 175, 111474. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, Y.; Xu, F.; Shao, X. The combined effects of tea tree oil and hot air treatment on the quality and sensory characteristics and decay of strawberry. Postharvest Biol. Technol. 2018, 136, 139–144. [Google Scholar] [CrossRef]
- Goñi, M.; Tomadoni, B.; Moreira, M.; Roura, S. Application of tea tree and clove essential oil on late development stages of Butterhead lettuce: Impact on microbiological quality. LWT Food Sci. Tech. 2013, 54, 107–113. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Ramachandran, C.; Hu, X.; Oh, D.-H.; Wang, M.-H. Chitosan-tea tree oil nanoemulsion and calcium chloride tailored edible coating increase the shelf life of fresh cut red bell pepper. Prog. Org. Coat. 2021, 151, 106010. [Google Scholar] [CrossRef]
- Servili, A.; Feliziani, E.; Romanazzi, G. Exposure to volatiles of essential oils alone or under hypobaric treatment to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2017, 133, 36–40. [Google Scholar] [CrossRef]
- Chaemsanit, S.; Matan, N.; Matan, N. Effect of peppermint oil on the shelf-life of dragon fruit during storage. Food Control 2018, 90, 172–179. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Garchery, C.; Stevens, R. Irrigation with salt water affects growth, yield, fruit quality, storability and marker-gene expression in cherry tomato. Acta Agric. Scand. Sect. B Plant Soil Sci. 2018, 68, 727–737. [Google Scholar] [CrossRef]
- Abdelgawad, K.F.; El-Mogy, M.M.; Mohamed, M.I.A.; Garchery, C.; Stevens, R.G. Increasing Ascorbic Acid Content and Salinity Tolerance of Cherry Tomato Plants by Suppressed Expression of the Ascorbate Oxidase Gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Yuan, C.; Chen, Y.; Li, H.; Liu, J. Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci. Hortic. 2014, 176, 45–53. [Google Scholar] [CrossRef]
- Sikora, M.; Świeca, M. Effect of ascorbic acid postharvest treatment on enzymatic browning, phenolics and antioxidant capacity of stored mung bean sprouts. Food Chem. 2018, 239, 1160–1166. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Parmar, A.; Ali, M.R.; Abdel-Aziz, M.E.; Abdeldaym, E.A. Improving postharvest storage of fresh artichoke bottoms by an edible coating of Cordia myxa gum. Postharvest Biol. Technol. 2020, 163, 111143. [Google Scholar] [CrossRef]
- Wang, K.; Cao, S.; Di, Y.; Liao, Y.; Zheng, Y. Effect of ethanol treatment on disease resistance against anthracnose rot in postharvest loquat fruit. Sci. Hortic. 2015, 188, 115–121. [Google Scholar] [CrossRef]
- Lichter, A.; Zutkhy, Y.; Sonego, L.; Dvir, O.; Kaplunov, T.; Sarig, P.; Ben-Arie, R. Ethanol controls postharvest decay of table grapes. Postharvest Biol. Technol. 2002, 24, 301–308. [Google Scholar] [CrossRef]
- Wang, K.; Jin, P.; Tang, S.; Shang, H.; Rui, H.; Di, H.; Cai, Y.; Zheng, Y. Improved control of postharvest decay in Chinese bayberries by a combination treatment of ethanol vapor with hot air. Food Control 2011, 22, 82–87. [Google Scholar] [CrossRef]
- Fukasawa, A.; Suzuki, Y.; Terai, H.; Yamauchi, N. Effects of postharvest ethanol vapor treatment on activities and gene expression of chlorophyll catabolic enzymes in broccoli florets. Postharvest Biol. Technol. 2010, 55, 97–102. [Google Scholar] [CrossRef]
- Asoda, T.; Terai, H.; Kato, M.; Suzuki, Y. Effects of postharvest ethanol vapor treatment on ethylene responsiveness in broccoli. Postharvest Biol. Technol. 2009, 52, 216–220. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Lv, D.Q.; Liu, W.W.; Qi, H.Y.; Bai, X.H. Ethanol vapor treatment maintains postharvest storage quality and inhibits internal ethylene biosynthesis during storage of oriental sweet melons. Postharvest Biol. Technol. 2013, 86, 372–380. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nagata, Y. Postharvest ethanol vapor treatment of tomato fruit stimulates gene expression of ethylene biosynthetic enzymes and ripening related transcription factors, although it suppresses ripening. Postharvest Biol. Technol. 2019, 152, 118–126. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, B.; Liu, Y.; Dunn, J.; Guerola, P.M.; Tortajada, M.; Cao, Z.; Ji, P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef] [Green Version]
- El-Mogy, M.M.; Ali, M.R.; Darwish, O.S.; Rogers, H.J. Impact of salicylic acid, abscisic acid, and methyl jasmonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. J. Berry Res. 2019, 9, 333–348. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Ludlow, R.A.; Roberts, C.; Müller, C.T.; Rogers, H.J. Postharvest exogenous melatonin treatment of strawberry reduces postharvest spoilage but affects components of the aroma profile. J. Berry Res. 2019, 9, 297–307. [Google Scholar] [CrossRef]
- Mostafa, H.S.; Ali, M.R.; Mohamed, R.M. Production of a novel probiotic date juice with anti-proliferative activity against Hep-2 cancer cells. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemistry (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemistry (AOAC): Gaithersburg, MD, USA, 2000. [Google Scholar]
- Shehata, S.A.; Elmogy, M.; Mohamed, H.F.Y. Postharvest quality and nutrient contents of long sweet pepper enhanced by supplementary potassium foliar application. Int. J. Veg. Sci. 2018, 25, 196–209. [Google Scholar] [CrossRef]
- Shahi, N.; Min, B.; Bonsi, E.A. Microbial decontamination of fresh produce (Strawberry) using washing solutions. J. Food Res. 2015, 4, 128. [Google Scholar] [CrossRef] [Green Version]
- Vasile, C.; Sivertsvik, M.; Mitelut, A.C.; Brebu, M.A.; Stoleru, E.; Rosnes, J.T.; Tănase, E.E.; Khan, W.; Pamfil, D.; Cornea, C.P.; et al. Comparative Analysis of the Composition and Active Property Evaluation of Certain Essential Oils to Assess their Potential Applications in Active Food Packaging. Materials 2017, 10, 45. [Google Scholar] [CrossRef]
- Kokina, M.; Kalušević, A.; Nedović, V.; Nikšić, M.; Shamtsyan, M.; Šavikin, K.; Pljevljakušić, D.; Pantić, M.; Lević, S.; Salević, A. Characterization, Antioxidant and Antibacterial Activity of Essential Oils and Their Encapsulation into Biodegradable Material Followed by Freeze Drying. Food Technol. Biotechnol. 2019, 57, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Loose, M.; Pilger, E.; Wagenlehner, F. Anti-Bacterial Effects of Essential Oils Against Uropathogenic Bacteria. Antibiotics 2020, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and safety attributes on shredded carrots by using Origanum majorana and ascorbic acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- Shao, X.; Wang, H.; Xu, F.; Cheng, S. Effects and possible mechanisms of tea tree oil vapor treatment on the main disease in postharvest strawberry fruit. Postharvest Biol. Technol. 2013, 77, 94–101. [Google Scholar] [CrossRef]
- Owolabi, I.O.; Songsamoe, S.; Matan, N. Combined impact of peppermint oil and lime oil on Mangosteen (Garcinia Mangostana) fruit ripening and mold growth using closed system. Postharvest Biol. Technol. 2021, 175, 111488. [Google Scholar] [CrossRef]
- Misharina, T.A.; Samusenko, A.L. Antioxidant properties of essential oils from lemon, grapefruit, coriander, clove, and their mixtures. Appl. Biochem. Microbiol. 2008, 44, 438–442. [Google Scholar] [CrossRef]
- Hernández-López, G.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Barrera-Necha, L.L. Nanostructured chitosan edible coating loaded with α-pinene for the preservation of the postharvest quality of Capsicum annuum L. and Alternaria alternata control. Int. J. Biol. Macromol. 2020, 165, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.S.; Malik, A.U.; Anwar, R.; Anjum, M.A.; Nawaz, A.; Shafique, M.; Naz, S. Combined application of ascorbic and oxalic acids delays postharvest browning of litchi fruits under controlled atmosphere conditions. Food Chem. 2021, 350, 129277. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Wang, X.; Wang, G.; Zheng, J.; Abeysinghe, D.C.; Ferguson, I.B.; Chen, K. Ethanol vapour treatment alleviates postharvest decay and maintains fruit quality in Chinese bayberry. Postharvest Biol. Technol. 2007, 46, 195–198. [Google Scholar] [CrossRef]
- Barzegar, T.; Fateh, M.; Razavi, F. Enhancement of postharvest sensory quality and antioxidant capacity of sweet pepper fruits by foliar applying calcium lactate and ascorbic acid. Sci. Hortic. 2018, 241, 293–303. [Google Scholar] [CrossRef]
- Lin, L.; Li, Q.P.; Wang, B.G.; Cao, J.K.; Jiang, W.B. Inhibition of core browning in ‘Yali’ pear fruit by post-harvest treatment with ascorbic acid. J. Hortic. Sci. Biotechnol. 2007, 82, 397–402. [Google Scholar] [CrossRef]
- Aminifard, M.H.; Mohammadi, S. Essential oils to control Botrytis cinerea in vitro and in vivo on plum fruits. J. Sci. Food Agric. 2012, 93, 348–353. [Google Scholar] [CrossRef]
- Suzuki, Y.; Uji, T.; Terai, H. Inhibition of senescence in broccoli florets with ethanol vapor from alcohol powder. Postharvest Biol. Technol. 2004, 31, 177–182. [Google Scholar] [CrossRef]
- Saltveit, M.E.; Mencarelli, F. Inhibition of ethylene synthesis and action in ripening tomato fruit by ethanol vapors. J. Am. Soc. Hortic. Sci. 1988, 113, 572–576. [Google Scholar]
- Rosenqvist, E.; Van Kooten, O. Chlorophyll fluorescence: A general description and nomenclature. In Practical Applications of Chlorophyll Fluorescence in Plant Biology; DeEll, J.R., Toivonen, P.M.A., Eds.; Springer: Boston, MA, USA, 2003; pp. 31–77. [Google Scholar]
- Naeem, A.; Abbas, T.; Ali, T.M.; Hasnain, A. Effect of guar gum coatings containing essential oils on shelf life and nutritional quality of green-unripe mangoes during low temperature storage. Int. J. Biol. Macromol. 2018, 113, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Kumar, S.; Kumar, V.; Sharma, R. Chitosan nanoemulsions as advanced edible coatings for fruits and vegetables: Composition, fabrication and developments in last decade. Int. J. Biol. Macromol. 2020, 152, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [Green Version]
- Viacava, G.E.; Goyeneche, R.; Goñi, M.G.; Roura, S.I.; Agüero, M.V. Natural elicitors as preharvest treatments to improve postharvest quality of Butterhead lettuce. Sci. Hortic. 2018, 228, 145–152. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; AbdelGawad, K.F. Effect of Some Citrus Essential Oils on Post-Harvest Shelf Life and Physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Yin, C.; Huang, C.; Wang, J.; Liu, Y.; Lu, P.; Huang, L. Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Zhang, J.; Wang, X.; Lin, Q.; Liu, W.; Xie, X.; Wang, Z.; Guan, W. Effects of UV-C irradiation on the physiological and antioxidant responses of button mushrooms (Agaricus bisporus) during storage. Int. J. Food Sci. Technol. 2016, 51, 1502–1508. [Google Scholar] [CrossRef]
- Qu, T.; Li, B.; Huang, X.; Li, X.; Ding, Y.; Chen, J.; Tang, X. Effect of Peppermint Oil on the Storage Quality of White Button Mushrooms (Agaricus bisporus). Food Bioprocess Technol. 2020, 13, 404–418. [Google Scholar] [CrossRef]
- Yang, F.; Li, H.; Li, F.; Xin, Z.; Zhao, L.; Zheng, Y.; Hu, Q. Effect of Nano-Packing on Preservation Quality of Fresh Strawberry (Fragaria ananassa Duch. cv Fengxiang) during Storage at 4 °C. J. Food Sci. 2010, 75, C236–C240. [Google Scholar] [CrossRef]
- Lin, X.; Wang, L.; Hou, Y.; Zheng, Y.; Jin, P. A Combination of Melatonin and Ethanol Treatment Improves Postharvest Quality in Bitter Melon Fruit. Foods 2020, 9, 1376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, S.; Chen, M.; Li, J.; Huang, D.; Zhu, S. Synergistic effects of ascorbic acid and plant-derived ceramide to enhance storability and boost antioxidant systems of postharvest strawberries. J. Sci. Food Agric. 2019, 99, 6562–6571. [Google Scholar] [CrossRef]
- Piscopo, A.; Zappia, A.; Princi, M.P.; De Bruno, A.; Araniti, F.; Antonio, L.; Abenavoli, M.R.; Poiana, M. Quality of shredded carrots minimally processed by different dipping solutions. J. Food Sci. Technol. 2019, 56, 2584–2593. [Google Scholar] [CrossRef]
- Lurie, S.; Pesis, E.; Gadiyeva, O.; Feygenberg, O.; Ben-Arie, R.; Kaplunov, T.; Zutahy, Y.; Lichter, A. Modified ethanol atmosphere to control decay of table grapes during storage. Postharvest Biol. Technol. 2006, 42, 222–227. [Google Scholar] [CrossRef]
- Gabler, F.M.; Mansour, M.F.; Smilanick, J.L.; Mackey, B.E. Survival of spores of Rhizopus stolonifer, Aspergillus niger, Botrytis cinerea and Alternaria alternata after exposure to ethanol solutions at various temperatures. J. Appl. Microbiol. 2004, 96, 1354–1360. [Google Scholar] [CrossRef]
- Zappia, A.; De Bruno, A.; Piscopo, A.; Poiana, M. Physico-chemical and microbiological quality of ready-to-eat rocket (Eruca vesicaria L. Cav.) treated with organic acids during storage in dark and light conditions. Food Sci. Biotechnol. 2019, 28, 965–973. [Google Scholar] [CrossRef]
- Hashem, M.; Alamri, S.A.; Alqahtani, M.S.; AlShehri, S.R. A multiple volatile oil blend prolongs the shelf life of peach fruit and suppresses postharvest spoilage. Sci. Hortic. 2019, 251, 48–58. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, L.; An, P.; Qi, J.; Yu, X.; Lu, J.; Ren, X. Antifungal mechanisms of α-terpineol and terpene-4-alcohol as the critical components of Melaleuca alternifolia oil in the inhibition of rot disease caused by Aspergillus ochraceus in postharvest grapes. J. Appl. Microbiol. 2019, 126, 1161–1174. [Google Scholar] [CrossRef] [PubMed]



| Compound | Rt (min) * | (%) |
|---|---|---|
| Peppermint oil (PMO) | ||
| Bicyclo [3.1.0]hex-2-ene, 4-methyl-1-(1-methylethyl)- | 8.17 | 1.3 |
| Bicyclo [3.1.0]hexane, 4-methylene-1-(1-methylethyl)- | 9.592 | 1.97 |
| D-Limonene | 11.43 | 11.19 |
| Decanal | 11.53 | 2.41 |
| Isopulegol | 15.63 | 1.67 |
| Cyclohexanone, 5-methyl-2-(1-methylethyl)-, cis- | 15.92 | 25.32 |
| Cyclohexanone, 5-methyl-2-(1-methylethyl)-, trans- | 16.3 | 14.68 |
| Levomenthol | 16.6 | 36.27 |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1.alpha.,2.alpha.,5.beta.)- | 20.84 | 5.19 |
| Tea tree oil (TTO) | ||
| Alpha.-Pinene, (-)- | 8.16 | 5.71 |
| (-)-.beta.-Pinene | 9.58 | 2.55 |
| Alpha.-Terpinene | 11.00 | 10.77 |
| Benzene, 1-methyl-3-(1-methylethyl)- | 11.29 | 6.36 |
| D-Limonene | 11.43 | 3.24 |
| Eucalyptol | 11.50 | 2.06 |
| Gamma.-Terpinene | 12.53 | 10.12 |
| Alpha.-Terpinolene | 13.59 | 3.60 |
| trans-β-Terpineol | 15.60 | 0.49 |
| 4-Terpinenol | 16.76 | 42.56 |
| Terpineol | 17.23 | 7.91 |
| γ-Terpineol | 17.48 | 1.43 |
| Caryophyllene | 24.90 | 3.19 |
| Mould and Yeast (CFU/g) | |||||
|---|---|---|---|---|---|
| 3 d | 6 d | 9 d | 12 d | 15 d | |
| Ethanol | ND * | ND | ND | ND | 1.35 ± 0.03 c |
| AsA | ND | 1.28 ± 0.12 b | 1.37 ± 0.03 b | 1.48 ± 0.09 ab | 1.32 ± 0.02 c |
| PMO | ND | ND | ND | 1.44 ± 0.03 b | 1.49 ± 0.01 b |
| TTO | ND | ND | ND | 1.41 ± 0.03 b | 1.37 ± 0.02 c |
| Control | ND | 1.54 ± 0.04 a | 1.66 ± 0.01 a | 1.68 ± 0.01 a | 1.79 ± 0.03 a |
| Total count (CFU/g) | |||||
| 3 d | 6 d | 9 d | 12 d | 15 d | |
| Ethanol | ND | ND | ND | ND | 0.53 ± 0.03 d |
| AsA | ND | ND | 0.63 ± 0.06 b | 0.90 ± 0.06 c | 1.30 ± 0.05 c |
| PMO | ND | ND | ND | 1.47 ± 0.01 b | 1.49 ± 0.01 b |
| TTO | ND | ND | ND | 1.55 ± 0.02 b | 1.56 ± 0.04 b |
| Control | ND | 1.71 ± 0.01 a | 1.76 ± 0.03 a | 1.93 ± 0.02 a | 2.05 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, A.H.R.; Parmar, A.; Ali, M.R.; El-Mogy, M.M.; Abdelgawad, K.F. Extending the Shelf-Life of Fresh-Cut Green Bean Pods by Ethanol, Ascorbic Acid, and Essential Oils. Foods 2021, 10, 1103. https://doi.org/10.3390/foods10051103
Awad AHR, Parmar A, Ali MR, El-Mogy MM, Abdelgawad KF. Extending the Shelf-Life of Fresh-Cut Green Bean Pods by Ethanol, Ascorbic Acid, and Essential Oils. Foods. 2021; 10(5):1103. https://doi.org/10.3390/foods10051103
Chicago/Turabian StyleAwad, Asmaa H. R., Aditya Parmar, Marwa R. Ali, Mohamed M. El-Mogy, and Karima F. Abdelgawad. 2021. "Extending the Shelf-Life of Fresh-Cut Green Bean Pods by Ethanol, Ascorbic Acid, and Essential Oils" Foods 10, no. 5: 1103. https://doi.org/10.3390/foods10051103