Antimicrobial Activity of Fermented Vegetable Byproduct Extracts for Food Applications
Abstract
:1. Introduction
2. Materials and Methods
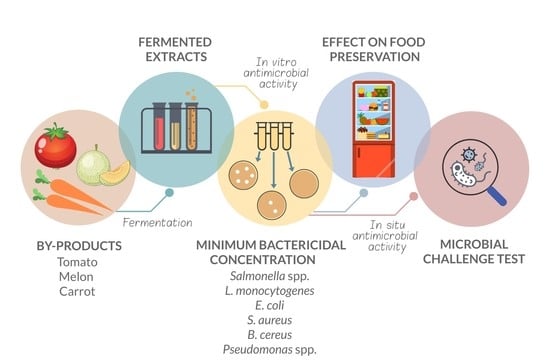
2.1. Byproduct Fermentation and Extract Production
2.2. Microorganisms Tested
2.3. Susceptibility Tests
2.4. Effect of Fermented Byproduct Extracts on Food Preservation
2.5. Effect of Fermented Tomato Byproduct Extracts on Foodborne Pathogens in Minced Meat
2.6. Statistical Analysis
3. Results
3.1. Minimum Bactericidal Concentration
3.2. Effect of Fermented Byproduct Extracts on Food Preservation
3.3. Effect of Fermented Tomato Byproduct Extract on Foodborne Pathogens in Minced Meat
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; I. World Health Organization: Geneva, Switzerland, 2015; p. 255. ISBN 978 92 4 156516 5. [Google Scholar]
- Pigłowski, M. Pathogenic and Non-Pathogenic Microorganisms in the Rapid Alert System for Food and Feed. Int. J. Environ. Res. Public Health 2019, 16, 477. [Google Scholar] [CrossRef] [Green Version]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Koutsoumanis, K.; Allende, A.; Alvarez-Ordo-nez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Guidance on date marking and related food information: Part 1 (date marking). EFSA J. 2020, 18, 630. [Google Scholar]
- Raposo, A.; Pérez, E.; Tinoco de Faria, C.; Ferrús, M.A.; Carrascosa, C. Food Spoilage by Pseudomonas spp.—An Overview. In Foodborne Pathogens and Antibiotic Resistance; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2017; Chapter 3; ISBN 9781119139157. [Google Scholar]
- David, J.R.D.; Steenson, L.R.; Davidson, P.M. Expectations and Applications of Natural Antimicrobials to Foods: A Guidance Document for Users, Suppliers, Research and Development, and Regulatory Agencies. Food Prot. Trends 2013, 33, 238–247. [Google Scholar]
- Nazir, F.; Salim, R.; Yousf, N.; Bashir, M.; Naik, H.R.; Hussain, S.Z. Natural antimicrobials for food preservation. J. Pharm. Phytochem. 2017, 6, 2078–2082. [Google Scholar]
- Tajkarimi, M.M.; Ibrahima, S.A.; Cliverb, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Ramos, L.; Moreno, C.; Zúñiga-Paredes, J.C.; Carlosama-Yepez, M.; Ruales, P. Antimicrobial activity of plant-food by-products: A review focusing on the tropics. Livest. Sci. 2016, 189, 32–49. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Int. Food Res. J. 2003, 2, 117–122. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Montanari, C.; Tabanelli, G.; Bargossi, E.; Gardini, F.; Lanciotti, R. Characterization of oregano (Origanum vulgare) essential oil and definition of its antimicrobial activity against Listeria monocytogenes and Escherichia coli in vitro system and on foodstuff surfaces. Afr. J. Microbiol. Res. 2014, 8, 2746–2753. [Google Scholar]
- Chai, T.T.; Tan, Y.N.; Ee, K.Y.; Xiao, J.; Wong, F.C. Seeds, fermented foods, and agricultural by-products as sources of plant-derived antibacterial peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, S162–S177. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Andrade, M.J.; Kylli, P.; Estévez, M. Avocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef]
- Ricci, A.; Bernini, V.; Maoloni, A.; Cirlini, M.; Galaverna, G.; Neviani, E.; Lazzi, C. Vegetable By-Product Lacto-Fermentation as a New Source of Antimicrobial Compounds. Microorganisms 2019, 7, 607. [Google Scholar] [CrossRef] [Green Version]
- Kothari, D.; Lee, W.D.; Jung, E.S.; Niu, K.M.; Lee, C.H.; Kim, S.K. Controlled Fermentation Using Autochthonous Lactobacillus plantarum Improves Antimicrobial Potential of Chinese Chives against Poultry Pathogens. Antibiotics 2020, 9, 386. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, G.; Chen, W. Beneficial Effects of Tomato Juice Fermented by Lactobacillus plantarum and Lactobacillus casei: Antioxidation, Antimicrobial Effect, and Volatile Profiles. Molecules 2018, 23, 2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Moving forward on Food Loss and Waste Reduction. In The State of Food and Agriculture 2019; FAO: Rome, Italy, 2019; ISBN 978-92-5-131789-1. [Google Scholar]
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.H.; Kwon, E.E.; Jeon, Y.J. Production of bioplastic through food waste valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Erika Coppo, E.; Ramona Barbieri, R.; Debbia, E.A.; Marchese, A. The effect of sub-inhibitory concentrations of rifaximin on urease production and on other virulence factors expressed by Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa and Staphylococcus aureus. J. Chemother. 2016, 29, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Becerril, R.; Manso, S.; Nerin, C.; Gómez-Lus, R. Antimicrobial activity of Lauroyl Arginate Ethyl (LAE), against selected food-borne bacteria. Food Control 2013, 32, 404–408. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Salomskiene, J.; Jonkuviene, D.; Macioniene, I.; Abraitiene, A.; Zeime, J.; Repeckiene, J.; Vaiciulyte-Funk, L. Differences in the occurence and efficiency of antimicrobial compounds produced by lactic acid bacteria. Eur. Food Res. Technol. 2019, 245, 569–579. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Calani, L.; Bernini, V.; Neviani, E.; Del Rio, D.; Galaverna, G.; Lazzi, C. In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem. 2019, 276, 692–699. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Maoloni, A.; Del Rio, D.; Calani, L.; Bernini, V.; Galaverna, G.; Neviani, E.; Lazzi, C. Use of Dairy and Plant-Derived Lactobacilli as Starters for Cherry Juice Fermentation. Nutrients 2019, 11, 213. [Google Scholar] [CrossRef] [Green Version]
- Valerio, F.; Lavermicocca, P.; Pascale, M.; Visconti, A. Production of phenyllactic acid by lactic acid bacteria: An approach to the selection of strains contributing to food quality and preservation. FEMS Microbiol. Lett. 2004, 233, 289–295. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Visconti, A. Antifungal Activity of Phenyllactic Acid against Molds Isolated from Bakery Products. Appl. Environ. Microbiol. 2003, 69, 634–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baydar, N.G.; Sagdic, O.; Ozkan, G. Determination of antibacterial effects and total phenolic contents of grape (Vitis vinifera L.) seed extracts. Int. J. Food Sci. 2006, 41, 799–804. [Google Scholar] [CrossRef]
- Hoque, M.M.; Bari, M.L.; Juneja, V.K.; Kawamoto, S. Antimicrobial activity of cloves and cinnamon extracts against food borne pathogens and spoilage bacteria and inactivation of Listeria monocytogenes in ground chicken meat with their essential oils. Rep. Natl. Food Res. Inst. 2008, 72, 9–21. [Google Scholar]
- Kramer, B.; Thielmann, J.; Hickisch, A.; Muranyi, P.; Wunderlich, J.; Hauser, C. Antimicrobial activity of hop extracts against foodborne pathogens for meat applications. J. Appl. Microbiol. 2015, 118, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; McKeever, L.C.; Malik, N.S. Assessment of the Antimicrobial Activity of Olive Leaf Extract against Foodborne Bacterial Pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef] [Green Version]
- Salvat, A.; Antonnacci, L.; Fortunato, R.H.; Suarez, E.Y.; Godoy, H.M. Screening of some plants from Northern Argentina for their antimicrobial activity. Lett. Appl. Microbiol. 2001, 32, 293–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Onofrio, V.; Gesuele, R.; Maione, A.; Liguori, G.; Liguori, R.; Guida, M.; Nigro, R.; Galdiero, E. Prevention of Pseudomonas aeruginosa Biofilm Formation on Soft Contact Lenses by Allium sativum Fermented Extract (BGE) and Cannabinol Oil Extract (CBD). Antibiotics 2019, 8, 258. [Google Scholar] [CrossRef] [Green Version]
- Yim, M.H.; Hong, T.G.; Lee, J.H. Antioxidant and Antimicrobial Activities of Fermentation and Ethanol Extracts of Pine Needles (Pinus densiflora). Food Sci. Biotechnol. 2006, 15, 582–588. [Google Scholar]
- He, X.; Zou, Y.; Yoon, W.B.; Park, S.J.; Park, D.S.; Ahn, J. Effects of probiotic fermentation on the enhancement of biological and pharmacological activities of Codonopsis lanceolata extracted by high pressure treatment. J. Biosci. Bioeng. 2011, 112, 188–193. [Google Scholar] [CrossRef]
- Eom, S.J.; Hwang, J.E.; Jung, J.; Jee, H.S.; Kim, K.-T.; Paik, H.D. Antioxidative and antibacterial activities on Staphylococcus aureus and Escherichia coli O157:H4 in milk with added ginseng marc extract fermented by Lactobacillus plantarum KCCM 11613P. J. Dairy Sci. 2017, 100, 7788–7792. [Google Scholar] [CrossRef]
- Sansawat, T.; Zhang, L.; Jeong, J.Y.; Xu, Y.; Hessell, G.W.; Ryser, E.T.; Harte, J.B.; Tempelman, R.; Kang, I. Inhibition of Listeria monocytogenes in full- and low-sodium frankfurters at 4, 7, or 10 °C using spray-dried mixtures of organic acid salts. J. Food Prot. 2013, 76, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Stobnicka, A.; Gniewosz, M. Antimicrobial protection of minced pork meat with the use of Swamp Cranberry (Vaccinium oxycoccos L.) fruit and pomace extracts. J. Food Sci. Technol. 2018, 55, 62–71. [Google Scholar] [CrossRef]
- Soriano, A.; Alañón, M.E.; Alarcón, M.; García-Ruíz, A.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Oak wood extracts as natural antioxidants to increase shelf life of raw pork patties in modified atmosphere packaging. Int. Food Res. J. 2018, 111, 524–533. [Google Scholar] [CrossRef]
- Nowak, A.; Czyzowska, A.; Efenberger, M.; Krala, L. Polyphenolic extracts of cherry (Prunus cerasus L.) and blackcurrant (Ribes nigrum L.) leaves as natural preservatives in meat products. Food Microbiol. 2016, 59, 142–149. [Google Scholar] [CrossRef]




| Tomato Byproduct Fermented Extract | Melon Byproduct Fermented Extract | Carrot Byproduct Fermented Extract | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microorganism | 8 log10 | 6 log10 | 4 log10 | 2 log10 | 8 log10 | 6 log10 | 4 log10 | 2 log10 | 8 log10 | 6 log10 | 4 log10 | 2 log10 |
| Salmonella ATCC 1408 | 25 | 25 | 25 | 12.5 | 25 | 25 | 25 | 25 | 12.5 | 12.5 | 12.5 | 12.5 |
| Salmonella Rissen | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 50 | 6.25 | 6.25 | 6.25 |
| Salmonella suini | 50 | 50 | 50 | 25 | 25 | 25 | 25 | 25 | 50 | 12.5 | 12.5 | 12.5 |
| L. monocytogenes LM30 | 50 | 25 | 25 | 12.5 | 25 | 25 | 25 | 25 | 25 | 12.5 | 12.5 | 6.25 |
| L. monocytogenes LMG 21264 | 100 | 25 | 25 | 12.5 | 25 | 25 | 25 | 12.5 | >50 | 25 | 6.25 | 6.25 |
| L. monocytogenes LMG 13305 | 100 | 25 | 25 | 12.5 | 25 | 25 | 25 | 25 | 50 | 25 | 12.5 | 6.25 |
| E. coli DSM 9025 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 6.25 | 6.25 | 6.25 |
| E. coli DSM 10973 | 25 | 25 | 25 | 12.5 | 25 | 25 | 25 | 25 | 25 | 6.25 | 6.25 | 6.25 |
| E. coli POM 1048 | 25 | 25 | 25 | 12.5 | 50 | 25 | 25 | 25 | 12.5 | 12.5 | 12.5 | 6.25 |
| S. aureus 9393 | 25 | 25 | 25 | 12.5 | 25 | 25 | 25 | 25 | 12.5 | 12.5 | 12.5 | 6.25 |
| S. aureus 6538 | 25 | 25 | 12.5 | 12.5 | 25 | 25 | 25 | 25 | 12.5 | 12.5 | 12.5 | 3.13 |
| S. aureus ATCC 19095 | 12.5 | 12.5 | 12.5 | 12.5 | 25 | 25 | 25 | 25 | 12.5 | 12.5 | 12.5 | 6.25 |
| B. cereus 31 | 25 | 25 | 25 | 12.5 | 25 | 25 | 25 | 25 | 12.5 | 12.5 | 12.5 | 6.25 |
| Pseudomonas spp. 5003 | 25 | 25 | 25 | 12.5 | 50 | 50 | 50 | 25 | 12.5 | 12.5 | 6.25 | 6.25 |
| Pseudomonas spp. 5004 | 25 | 25 | 25 | 12.5 | 50 | 50 | 25 | 12.5 | 25 | 6.25 | 6.25 | 6.25 |
| Pseudomonas spp. 5005 | 25 | 25 | 25 | 12.5 | 25 | 25 | 25 | 25 | 12.5 | 6.25 | 6.25 | 6.25 |
| T0 | T7 | T15 | T30 | |
|---|---|---|---|---|
| Ready-to-eat sliced vegetal product | 4.04 ± 0.47 | 4.12 ± 0.06 | 7.22 ± 0.07 | 6.48 ± 0.16 |
| Ready-to-eat sliced vegetal product + fermented melon byproduct extract | <1 | <1 | <1 | <1 |
| Ready-to-eat sliced vegetal product + fermented carrot byproduct extract | <1 | <1 | 1.83 ± 0.40 | 1.44 ± 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, A.; Bertani, G.; Maoloni, A.; Bernini, V.; Levante, A.; Neviani, E.; Lazzi, C. Antimicrobial Activity of Fermented Vegetable Byproduct Extracts for Food Applications. Foods 2021, 10, 1092. https://doi.org/10.3390/foods10051092
Ricci A, Bertani G, Maoloni A, Bernini V, Levante A, Neviani E, Lazzi C. Antimicrobial Activity of Fermented Vegetable Byproduct Extracts for Food Applications. Foods. 2021; 10(5):1092. https://doi.org/10.3390/foods10051092
Chicago/Turabian StyleRicci, Annalisa, Gaia Bertani, Antonietta Maoloni, Valentina Bernini, Alessia Levante, Erasmo Neviani, and Camilla Lazzi. 2021. "Antimicrobial Activity of Fermented Vegetable Byproduct Extracts for Food Applications" Foods 10, no. 5: 1092. https://doi.org/10.3390/foods10051092