Effects of Extraction Conditions on Banana Peel Polyphenol Oxidase Activity and Insights into Inactivation Kinetics Using Thermal and Cold Plasma Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Reagents
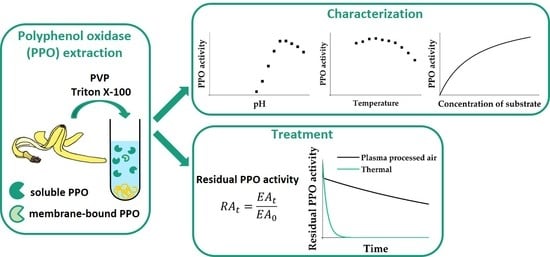
2.2. Preparation of Crude Enzyme Extracts
2.3. Fractionation of Crude Enzyme Extracts
2.4. Protein Determination of Crude Enzyme Extracts
2.5. PPO Activity Assay
2.6. Characterization of Crude PPO
2.7. Thermal PPO Inactivation in Crude Enzyme Extracts
2.8. Plasma Treatment of Crude Enzyme Extracts
2.9. Kinetic Data Analysis
2.10. Statistical Analyses
3. Results
3.1. Adaption of PPO Extraction and Characterization of Temperature and pH Optimum of ‘Prata’ Banana Peel PPO
3.1.1. Adaption of PPO Extraction
3.1.2. Temperature and pH Optimum of Crude ‘Prata’ Banana Peel PPO
3.1.3. Substrate Specificity and Kinetic Parameters—Oxidation of Phenolic Compounds
3.1.4. Substrate Specificity and Kinetic Parameters—Adduct Formation of 4-Methylcatechol and L-Proline
3.2. Temperature Stability of Crude Banana Peel PPO
3.3. Kinetics of Crude PPO Thermal Inactivation
3.4. Thermal Inactivation Kinetics of Membrane-Bound and Soluble PPO
3.5. Effects of Plasma Treatment on PPO Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soto, M. Situación y avances tecnológicos en la producción bananera mundial. Rev. Bras. De Frutic. 2011, 33, 13–28. [Google Scholar] [CrossRef]
- Happi Emaga, T.; Andrianaivo, R.H.; Wathelet, B.; Tchango, J.T.; Paquot, M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007, 103, 590–600. [Google Scholar] [CrossRef]
- Schieber, A. Side streams of plant food processing as a source of valuable compounds: Selected examples. Annu. Rev. Food Sci. Technol. 2017, 8, 97–112. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic compounds within banana peel and their potential uses: A review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Yang, C.-P.; Fujita, S.; Kohno, K.; Kusubayashi, A.; Ashrafuzzaman, M.; Hayashi, N. Partial purification and characterization of polyphenol oxidase from banana (musa sapientum L.) peel. J. Agric. Food Chem. 2001, 49, 1446–1449. [Google Scholar] [CrossRef]
- Zaini, N.A.M.; Osman, A.; Hamid, A.A.; Ebrahimpour, A.; Saari, N. Purification and characterization of membrane-bound polyphenoloxidase (mppo) from snake fruit [salacca zalacca (gaertn.) voss]. Food Chem. 2013, 136, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.A.; Mohan, T.C.K.; Murugan, K. Purification and kinetic characterization of polyphenol oxidase from barbados cherry (Malpighia glabra L.). Food Chem. 2008, 110, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Marques Silva, F.V.; Sulaiman, A. Polyphenoloxidase in fruit and vegetables: Inactivation by thermal and non-thermal processes. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 287–301. [Google Scholar]
- Terefe, N.S.; Delon, A.; Buckow, R.; Versteeg, C. Blueberry polyphenol oxidase: Characterization and the kinetics of thermal and high pressure activation and inactivation. Food Chem. 2015, 188, 193–200. [Google Scholar] [CrossRef]
- Surowsky, B.; Schlüter, O.; Knorr, D. Interactions of non-thermal atmospheric pressure plasma with solid and liquid food systems: A review. Food Eng. Rev. 2015, 7, 82–108. [Google Scholar] [CrossRef]
- Kramer, B.; Hasse, D.; Guist, S.; Schmitt-John, T.; Muranyi, P. Inactivation of bacterial endospores on surfaces by plasma processed air. J. Appl. Microbiol. 2020, 128, 920–933. [Google Scholar] [CrossRef] [Green Version]
- Chutia, H.; Kalita, D.; Mahanta, C.L.; Ojah, N.; Choudhury, A.J. Kinetics of inactivation of peroxidase and polyphenol oxidase in tender coconut water by dielectric barrier discharge plasma. LWT 2019, 101, 625–629. [Google Scholar] [CrossRef]
- Tappi, S.; Berardinelli, A.; Ragni, L.; Dalla Rosa, M.; Guarnieri, A.; Rocculi, P. Atmospheric gas plasma treatment of fresh-cut apples. Innov. Food Sci. Emerg. Technol. 2014, 21, 114–122. [Google Scholar] [CrossRef]
- Bußler, S.; Ehlbeck, J.; Schlüter, O.K. Pre-drying treatment of plant related tissues using plasma processed air: Impact on enzyme activity and quality attributes of cut apple and potato. Innov. Food Sci. Emerg. Technol. 2017, 40, 78–86. [Google Scholar] [CrossRef]
- Ünal, M.Ü. Properties of polyphenol oxidase from anamur banana (Musa cavendishii). Food Chem. 2007, 100, 909–913. [Google Scholar] [CrossRef]
- Chaisakdanugull, C.; Theerakulkait, C. Partial purification and characterisation of banana [Musa (aaa group) ‘Gros Michel’] polyphenol oxidase. Int. J. Food Sci. Technol. 2009, 44, 840–846. [Google Scholar] [CrossRef]
- Yang, C.-P.; Fujita, S.; Ashrafuzzaman, M.; Nakamura, N.; Hayashi, N. Purification and characterization of polyphenol oxidase from banana (Musa sapientum L.) pulp. J. Agric. Food Chem. 2000, 48, 2732–2735. [Google Scholar] [CrossRef]
- Galeazzi, M.A.M.; Sgarbieri, V.C. Substrate specificity and inhibition of polyphenoloxidase (PPO) from a dwarf variety of banana (Musa cavendishii, L.). J. Food Sci. 1981, 46, 1404–1406. [Google Scholar] [CrossRef]
- Galeazzi, M.A.M.; Sgarbieri, V.C.; Constantinides, S.M. Isolation, purification and physicochemical characterization of polyphenoloxidases (PPO) from a dwarf variety of banana (Musa cavendishii, L.). J. Food Sci. 1981, 46, 150–155. [Google Scholar] [CrossRef]
- Ngalani, J.; Signoret, A.; Crouzet, J. Partial purification and properties of plantain polyphenol oxidase. Food Chem. 1993, 48, 341–347. [Google Scholar] [CrossRef]
- Padrón, M.P.; Lozano, J.A.; González, A.G. Properties of o-diphenol: O2 oxidoreductase from Musa cavendishii. Phytochemistry 1975, 14, 1959–1963. [Google Scholar] [CrossRef]
- Montgomery, M.W.; Sgarbieri, V.C. Isoenzymes of banana polyphenol oxidase. Phytochemistry 1975, 14, 1245–1249. [Google Scholar] [CrossRef]
- Palmer, J.K. Banana polyphenoloxidase. Preparation and properties. Plant Physiol. 1963, 38, 508–513. [Google Scholar] [PubMed] [Green Version]
- Gómez-López, V.M. Some biochemical properties of polyphenol oxidase from two varieties of avocado. Food Chem. 2002, 77, 163–169. [Google Scholar] [CrossRef]
- Rocha, A.M.C.N.; Morais, A.M.M.B. Characterization of polyphenoloxidase (PPO) extracted from ‘Jonagored’ apple. Food Control 2001, 12, 85–90. [Google Scholar]
- Palma-Orozco, G.; Marrufo-Hernández, N.A.; Sampedro, J.G.; Nájera, H. Purification and partial biochemical characterization of polyphenol oxidase from mango (Mangifera indica cv. Manila). J. Agric. Food Chem. 2014, 62, 9832–9840. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S.; Sharma, A. Purification and characterisation of polyphenol oxidase (PPO) from eggplant (Solanum melongena). Food Chem. 2012, 134, 1855–1861. [Google Scholar] [PubMed]
- Zhou, L.; Liu, W.; Terefe, N.S. The inactivation kinetics of soluble and membrane-bound polyphenol oxidase in pear during thermal and high-pressure processing. Food Bioprocess Technol. 2018, 11, 1039–1049. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Schweiggert, U.; Schieber, A.; Carle, R. Inactivation of peroxidase, polyphenoloxidase, and lipoxygenase in paprika and chili powder after immediate thermal treatment of the plant material. Innov. Food Sci. Emerg. Technol. 2005, 6, 403–411. [Google Scholar] [CrossRef]
- Tan, T.-C.; Cheng, L.-H.; Bhat, R.; Rusul, G.; Easa, A.M. Composition, physicochemical properties and thermal inactivation kinetics of polyphenol oxidase and peroxidase from coconut (Cocos nucifera) water obtained from immature, mature and overly-mature coconut. Food Chem. 2014, 142, 121–128. [Google Scholar] [CrossRef]
- Goyeneche, R.; Di Scala, K.; Roura, S. Biochemical characterization and thermal inactivation of polyphenol oxidase from radish (Raphanus sativus var. Sativus). LWT Food Sci. Technol. 2013, 54, 57–62. [Google Scholar] [CrossRef]
- Illera, A.E.; Chaple, S.; Sanz, M.T.; Ng, S.; Lu, P.; Jones, J.; Carey, E.; Bourke, P. Effect of cold plasma on polyphenol oxidase inactivation in cloudy apple juice and on the quality parameters of the juice during storage. Food Chem. 2019, 3, 100049. [Google Scholar] [CrossRef]
- Jaramillo Sánchez, G.M.; Garcia Loredo, A.B.; Contigiani, E.V.; Gómez, P.L.; Alzamora, S.M. Inactivation kinetics of peroxidase and polyphenol oxidase in peach juice treated with gaseous ozone. Int. J. Food Sci. Technol. 2018, 53, 347–355. [Google Scholar] [CrossRef]
- Sulaiman, A.; Soo, M.J.; Yoon, M.M.; Farid, M.; Silva, F.V. Modeling the polyphenoloxidase inactivation kinetics in pear, apple and strawberry purees after high pressure processing. J. Food Eng. 2015, 147, 89–94. [Google Scholar] [CrossRef]
- Loomis, W.D.; Battaile, J. Plant phenolic compounds and the isolation of plant enzymes. Phytochemistry 1966, 5, 423–438. [Google Scholar] [CrossRef]
- Wuyts, N.; De Waele, D.; Swennen, R. Extraction and partial characterization of polyphenol oxidase from banana (Musa acuminata Grande Naine) roots. Plant Physiol. Biochem. 2006, 44, 308–314. [Google Scholar] [CrossRef]
- Griffiths, L.A. Detection and identification of the polyphenoloxidase substrate of the banana. Nature 1959, 184, 58–59. [Google Scholar] [CrossRef]
- Rzepecki, L.M.; Waite, J.H. A chromogenic assay for catecholoxidases based on the addition of L-proline to quinones. Anal. Biochem. 1989, 179, 375–381. [Google Scholar] [CrossRef]
- Terefe, N.S.; Delon, A.; Versteeg, C. Thermal and high pressure inactivation kinetics of blueberry peroxidase. Food Chem. 2017, 232, 820–826. [Google Scholar] [CrossRef]
- Han, Q.-Y.; Liu, F.; Li, M.; Wang, K.-L.; Ni, Y.-Y. Comparison of biochemical properties of membrane-bound and soluble polyphenol oxidase from granny smith apple (Malus × domestica Borkh.). Food Chem. 2019, 289, 657–663. [Google Scholar] [CrossRef]
- Surowsky, B.; Fischer, A.; Schlueter, O.; Knorr, D. Cold plasma effects on enzyme activity in a model food system. Innov. Food Sci. Emerg. Technol. 2013, 19, 146–152. [Google Scholar] [CrossRef]






| Series | Tested Parameters | Filter Aid (g) | Triton X (% (v/v)) | Liquid/Solid Ratio (mL/g) | Extraction Time (h) | Homogenization Time (min) | |
|---|---|---|---|---|---|---|---|
| PVP | PVPP | ||||||
| A | Added filter aid | 0, 0.5, 1 | 1, 2 | 0 | 30 | 4 | 3 |
| B | Added Triton X-100 | 0.5 | - | 0, 0.5, 1 | 30 | 4 | 3 |
| C | Liquid/solid ratio | 0.5 | - | 0.5 | 30, 40, 50 | 4 | 3 |
| D | Extraction time | 0.5 | - | 0.5 | 30 | 0, 2, 4, 8, 10, 24 | 3 |
| E | Homogenization time | 0.5 | - | 0.5 | 30 | 0 | 0, 3, 6 |
| Filter Aid | Triton X-100 | Liquid/Solid Ratio | Extraction Time | Homogenization Time | Volumetric PPO Activity | Specific PPO Activity |
|---|---|---|---|---|---|---|
| (g) | (% (v/v)) | (mL/g) | (h) | (min) | (nkat/mL) | (nkat/mgprotein) |
| Series A | ||||||
| - | - | 30 | 4 | 3 | 0.7 ± 0.1 a | 36.6 ± 7.7 a |
| 0.5 g PVP | - | 30 | 4 | 3 | 33.5 ± 4.0 b | 176.5 ± 19.1 b |
| 1 g PVP | - | 30 | 4 | 3 | 35.2 ± 5.1 b | 167.1 ± 15.8 b |
| 1 g PVPP d | - | 30 | 4 | 3 | 0.2 ± 0.2 a | 12.8 ± 14.7 a |
| 2 g PVPP | - | 30 | 4 | 3 | 0.2 ± 0.2 a | 17.1 ± 9.2 a |
| Series B | ||||||
| - | - | 30 | 4 | 3 | 0.8 ± 0.1 a | 46.5 ± 7.5 a |
| 0.5 g PVP | - | 30 | 4 | 3 | 26.5 ± 0.6 b | 143.9 ± 10.5 b |
| 0.5 g PVP | 0.5 | 30 | 4 | 3 | 58.4 ± 2.2 c | 156.0 ± 9.0 b |
| 0.5 g PVP | 1 | 30 | 4 | 3 | 64.7 ± 7.3 c | 149.5 ± 8.1 b |
| Series C | ||||||
| - | - | 30 | 4 | 3 | 1.1 ± 0.3 a | 66.8 ± 10.3 a |
| 0.5 g PVP | 0.5 | 30 | 4 | 3 | 54.8 ± 9.5 c | 143.9 ± 18.3 b |
| 0.5 g PVP | 0.5 | 40 | 4 | 3 | 42.8 ± 1.7 b,c | 128.4 ± 6.8 b |
| 0.5 g PVP | 0.5 | 50 | 4 | 3 | 31.0 ± 3.5 b | 123.1 ± 10.5 b |
| Series D | ||||||
| - | - | 30 | 4 | 3 | 0.6 ± 0.1 a | 31.9 ± 7.5 a |
| 0.5 g PVP | 0.5 | 30 | 0 | 3 | 57.1 ± 3.5 b | 165.7 ± 8.6 b |
| 0.5 g PVP | 0.5 | 30 | 2 | 3 | 57.6 ± 7.6 b | 168.5 ± 19.2 b |
| 0.5 g PVP | 0.5 | 30 | 4 | 3 | 58.4 ± 2.2 b | 156.0 ± 9.0 b |
| 0.5 g PVP | 0.5 | 30 | 8 | 3 | 53.9 ± 0.9 b | 151.8 ± 1.3 b |
| 0.5 g PVP | 0.5 | 30 | 10 | 3 | 54.0 ± 0.7 b | 154.6 ± 5.1 b |
| 0.5 g PVP | 0.5 | 30 | 24 | 3 | 52.1 ± 3.3 b | 122.7 ± 6.7 c |
| Series E | ||||||
| - | - | 30 | 4 | 3 | 0.9 ± 0.2 a,* | 56.2 ± 8.3 a,* |
| 0.5 g PVP | 0.5 | 30 | 0 | 0 | 47.5 ± 7.2 b | 143.6 ± 12.2 b |
| 0.5 g PVP | 0.5 | 30 | 0 | 3 | 50.1 ± 3.4 b | 135.4 ± 11.1 b |
| 0.5 g PVP | 0.5 | 30 | 0 | 6 | 42.7 ± 1.4 b | 119.3 ± 8.5 b |
| Substrate | Wavelength | Volumetric PPO Activity | KM | Vmax | Vmax/KM |
|---|---|---|---|---|---|
| (nm) | (Units/mL) * | (mM) | (Units) | (Units/mM) | |
| Dopamine | 475 | 1010.7 ± 6.4 | 0.94 ± 0.07 a | 104.5 ± 2.3 b | 110.8 ± 8.9 b |
| Chlorogenic acid | 400 | 55.8 ± 3.8 | 7.54 ± 0.51 c | 78.7 ± 2.9 a | 10.4 ± 0.8 a |
| L-tyrosine | 400 | 0 ± 0 | - | - | - |
| Model | Parameter | Estimated Values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PPO | mPPO | sPPO | |||||||
| 60 °C | 70 °C | 80 °C | 90 °C | 70 °C | 80 °C | 70 °C | 80 °C | ||
| First-order | k (min−1) | 0.014 ± 0.001 | 0.031 ± 0.002 | 0.091 ± 0.004 | 0.197 ± 0.007 | 0.042 ± 0.002 | 0.100 ± 0.003 | 0.107 ± 0.010 | 0.251 ± 0.007 |
| R2adj | 0.98 | 0.95 | 0.99 | 1.00 | 0.99 | 1.00 | 0.95 | 1.00 | |
| RMSE | 0.05 | 0.08 | 0.04 | 0.02 | 0.05 | 0.02 | 0.07 | 0.02 | |
| Residual plot | Patterned | Patterned | Patterned | ||||||
| Two-fraction | kL (min−1) | 0.177 ± 0.098 | 0.090 ± 0.023 | 0.090 ± 0.005 | 0.195 ± 0.008 | 0.060 ± 0.006 | 0.100 ± 0.004 | 0.290 ± 0.032 | 0.348 ± 0.522 |
| kS (min−1) | 0.011 ± 0.001 | 0.011 ± 0.002 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.007 ± 0.003 | 0.000 ± 0.000 | 0.026 ± 0.003 | 0.152 ± 0.246 | |
| α (-) | 0.151 ± 0.038 | 0.505 ± 0.081 | 1.000 ± 0.001 | 1.000 ± 0.007 | 0.810 ± 0.056 | 1.000 ± 0.008 | 0.633 ± 0.036 | 0.637 ± 1.701 | |
| R2adj | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| RMSE | 0.04 | 0.05 | 0.04 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wohlt, D.; Schwarz, E.; Schieber, A.; Bader-Mittermaier, S. Effects of Extraction Conditions on Banana Peel Polyphenol Oxidase Activity and Insights into Inactivation Kinetics Using Thermal and Cold Plasma Treatment. Foods 2021, 10, 1022. https://doi.org/10.3390/foods10051022
Wohlt D, Schwarz E, Schieber A, Bader-Mittermaier S. Effects of Extraction Conditions on Banana Peel Polyphenol Oxidase Activity and Insights into Inactivation Kinetics Using Thermal and Cold Plasma Treatment. Foods. 2021; 10(5):1022. https://doi.org/10.3390/foods10051022
Chicago/Turabian StyleWohlt, Daria, Elena Schwarz, Andreas Schieber, and Stephanie Bader-Mittermaier. 2021. "Effects of Extraction Conditions on Banana Peel Polyphenol Oxidase Activity and Insights into Inactivation Kinetics Using Thermal and Cold Plasma Treatment" Foods 10, no. 5: 1022. https://doi.org/10.3390/foods10051022