Composite Coatings of Chitosan and Alginate Emulsions with Olive Oil to Enhance Postharvest Quality and Shelf Life of Fresh Figs (Ficus carica L. cv. ‘Pingo De Mel’)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
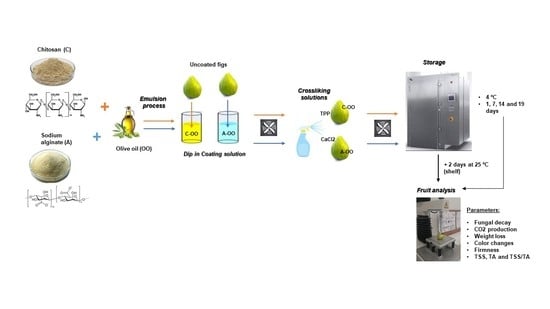
2.2. Coating Emulsions Preparation and Viscosity Evaluation
2.3. Sample Preparation and Storage Conditions
2.4. Analytical Control of Fruits
2.4.1. Fruit Fungal Infection and Disease Incidence
2.4.2. Respiration Rate
2.4.3. Weight Loss
2.4.4. Firmness and Surface Color
2.4.5. Total Soluble Solids and Titratable Acidity
2.5. Statistical Analysis and Adjusts
3. Results and Discussion
3.1. Viscosity Properties of the Coating Emulsions
3.1.1. Apparent Viscosity Curves
3.1.2. Effect of Temperature on Apparent Viscosity
3.2. Effect of Coatings Application on Figs Quality under Storage
3.2.1. Fruit Fungal Infections and Disease Incidence
3.2.2. Respiration Rate
3.2.3. Weight Loss
3.2.4. Firmness and Surface Color
3.2.5. Total Soluble Solids and Titratable Acidity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamiloglu, S.; Capanoglu, E. Polyphenol Content in Figs (Ficus carica L.): Effect of Sun-Drying. Int. J. Food Prop. 2015, 18, 521–535. [Google Scholar] [CrossRef]
- Carlos, H.C.; Vanessa, B.; Louise, F.; Gayle, M.C. Evaluating Quality Attributes of Four Fresh Fig (Ficus carica L.) Cultivars Harvested at Two Maturity Stages. HortScience 2010, 45, 707–710. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Avalos, M.C.; Femenia, A.; Minjares-Fuentes, R.; Contreras-Esquivel, J.C.; Aguilar-González, C.N.; Esparza-Rivera, J.R.; Meza-Velázquez, J.A. Improvement of the Quality and the Shelf Life of Figs (Ficus carica) Using an Alginate–Chitosan Edible Film. Food Bioprocess Technol. 2016, 9, 2114–2124. [Google Scholar] [CrossRef]
- Allegra, A.; Gallotta, A.; Carimi, F.; Mercati, F.; Inglese, P.; Martinelli, F. Metabolic Profiling and Post-harvest Behavior of “Dottato” Fig (Ficus carica L.) Fruit Covered With an Edible Coating from O. ficus-indica. Front. Plant Sci. 2018, 9, 1321. [Google Scholar] [CrossRef]
- FAOSTAT. FAO Statistical Databe. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 2 January 2019).
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant Activities and Anthocyanin Content of Fresh Fruits of Common Fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef]
- Villalobos, M.d.C.; Serradilla, M.J.; Martín, A.; López Corrales, M.; Pereira, C.; Córdoba, M.d.G. Preservation of different fig cultivars (Ficus carica L.) under modified atmosphere packaging during cold storage. J. Sci. Food Agric. 2016, 96, 2103–2115. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Ku, V.V.V.; Shohet, D.; Kim, G.H. Importance of low ethylene levels to delay senescence of non-climacteric fruit and vegetables. Aust. J. Exp. Agric. 1999, 39, 221–224. [Google Scholar] [CrossRef]
- Martínez-García, J.J.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Ramírez-Baca, P.; Candelas-Cadillo, M.G.; González-Laredo, R.F. Drying Parameters of Half-Cut and Ground Figs (Ficus carica L.) var. Mission and the Effect on Their Functional Properties. J. Eng. 2013, 2013, 710830. [Google Scholar] [CrossRef] [Green Version]
- Villalobos, M.d.C.; Serradilla, M.J.; Martín, A.; Hernández-León, A.; Ruíz-Moyano, S.; Córdoba, M.d.G. Characterization of microbial population of breba and main crops (Ficus carica) during cold storage: Influence of passive modified atmospheres (MAP) and antimicrobial extract application. Food Microbiol. 2017, 63, 35–46. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings—A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Gheorghita, R.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Sanchez, A.; Villalonga Santana, R.; Mariniello, L. Effect of Mesoporous Silica Nanoparticles on The Physicochemical Properties of Pectin Packaging Material for Strawberry Wrapping. Nanomaterials 2020, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Senturk Parreidt, T.; Schmid, M.; Müller, K. Effect of Dipping and Vacuum Impregnation Coating Techniques with Alginate Based Coating on Physical Quality Parameters of Cantaloupe Melon. J. Food Sci. 2018, 83, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Senturk Parreidt, T.; Lindner, M.; Rothkopf, I.; Schmid, M.; Müller, K. The Development of a Uniform Alginate-Based Coating for Cantaloupe and Strawberries and the Characterization of Water Barrier Properties. Foods 2019, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Syarifuddin, A.; Dirpan, A.; Mahendradatta, M. Physical, mechanical, and barrier properties of sodium alginate/gelatin emulsion based-films incorporated with canola oil. IOP Conf. Ser. Earth Environ. Sci. 2017, 101, 012019. [Google Scholar] [CrossRef]
- Lin, M.G.; Lasekan, O.; Saari, N.; Khairunniza-Bejo, S. Effect of chitosan and carrageenan-based edible coatings on post-harvested longan (Dimocarpus longan) fruits. CyTA J. Food 2018, 16, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Pereira Dos Santos, E.; Nicácio, P.H.M.; Coêlho Barbosa, F.; Nunes da Silva, H.; Andrade, A.L.S.; Lia Fook, M.V.; de Lima Silva, S.M.; Farias Leite, I. Chitosan/Essential Oils Formulations for Potential Use as Wound Dressing: Physical and Antimicrobial Properties. Materials 2019, 12, 2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Jara, C.; Bilbao-Sainz, C.; McHugh, T.; Chiou, B.-S.; Williams, T.; Villalobos-Carvajal, R. Physical, mechanical and transport properties of emulsified films based on alginate with soybean oil: Effects of soybean oil concentration, number of passes and degree of surface crosslinking. Food Hydrocoll. 2020, 109, 106133. [Google Scholar] [CrossRef]
- Kingwascharapong, P.; Arisa, K.; Karnjanapratum, S.; Tanaka, F.; Tanaka, F. Effect of gelatin-based coating containing frog skin oil on the quality of persimmon and its characteristics. Sci. Hortic. 2020, 260, 108864. [Google Scholar] [CrossRef]
- Gutiérrez-Pacheco, M.M.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Lizardi-Mendoza, J.; Miranda, R.; Ayala-Zavala, J.F. Individual and Combined Coatings of Chitosan and Carnauba Wax with Oregano Essential Oil to Avoid Water Loss and Microbial Decay of Fresh Cucumber. Coatings 2020, 10, 614. [Google Scholar] [CrossRef]
- Reyes-Avalos, M.C.; Minjares-Fuentes, R.; Femenia, A.; Contreras-Esquivel, J.C.; Quintero-Ramos, A.; Esparza-Rivera, J.R.; Meza-Velázquez, J.A. Application of an Alginate–Chitosan Edible Film on Figs (Ficus carica): Effect on Bioactive Compounds and Antioxidant Capacity. Food Bioprocess Technol. 2019, 12, 499–511. [Google Scholar] [CrossRef]
- Vieira, T.M.; Moldão-Martins, M.; Alves, V.D. Development and characterization of crosslinked chitosan and alginate emulsion-based films as green packaging materials for food application. Foods 2021. under review. [Google Scholar]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Torres, C.A.V.; Ferreira, A.R.V.; Freitas, F.; Reis, M.A.M.; Coelhoso, I.; Sousa, I.; Alves, V.D. Rheological studies of the fucose-rich exopolysaccharide FucoPol. Int. J. Biol. Macromol. 2015, 79, 611–617. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemists Inc: Rockville, MD, USA, 2012. [Google Scholar]
- Nijenhuis, K.; McKinley, G.; Spiegelberg, S.; Barnes, H.; Aksel, N.; Heymann, L.; Odell, J. Non-Newtonian Flows. In Springer Handbook of Experimental Fluid Mechanics; Tropea, C., Yarin, A.L., Foss, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 619–743. [Google Scholar] [CrossRef]
- İbanoğlu, E. Rheological behaviour of whey protein stabilized emulsions in the presence of gum arabic. J. Food Eng. 2002, 52, 273–277. [Google Scholar] [CrossRef]
- Cruz, M.; Freitas, F.; Torres, C.A.V.; Reis, M.A.M.; Alves, V.D. Influence of temperature on the rheological behavior of a new fucose-containing bacterial exopolysaccharide. Int. J. Biol. Macromol. 2011, 48, 695–699. [Google Scholar] [CrossRef]
- Sittikijyothin, W.; Torres, D.; Gonçalves, M.P. Modelling the rheological behaviour of galactomannan aqueous solutions. Carbohydr. Polym. 2005, 59, 339–350. [Google Scholar] [CrossRef]
- Murillo-Martínez, M.M.; Pedroza-Islas, R.; Lobato-Calleros, C.; Martínez-Ferez, A.; Vernon-Carter, E.J. Designing W1/O/W2 double emulsions stabilized by protein–polysaccharide complexes for producing edible films: Rheological, mechanical and water vapour properties. Food Hydrocoll. 2011, 25, 577–585. [Google Scholar] [CrossRef]
- Purwanti, N.; Zehn, A.S.; Pusfitasari, E.D.; Khalid, N.; Febrianto, E.Y.; Mardjan, S.S.; Andreas; Kobayashi, I. Emulsion stability of clove oil in chitosan and sodium alginate matrix. Int. J. Food Prop. 2018, 21, 566–581. [Google Scholar] [CrossRef] [Green Version]
- Romanazzi, G.; Feliziani, E.; Santini, M.; Landi, L. Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biol. Technol. 2013, 75, 24–27. [Google Scholar] [CrossRef]
- Moayednia, N.; Ehsani, M.R.; Emamdjomeh, Z.; Asadi, M.M.; Mizani, M.; Mazaheri, A.F. A note on the effect of calcium alginate coating on quality of refrigerated strawberries. Ir. J. Agric. Food Res. 2010, 49, 165–170. [Google Scholar]
- Aparicio-García, P.F.; Ventura-Aguilar, R.I.; del Río-García, J.C.; Hernández-López, M.; Guillén-Sánchez, D.; Salazar-Piña, D.A.; Ramos-García, M.d.L.; Bautista-Baños, S. Edible Chitosan/Propolis Coatings and Their Effect on Ripening, Development of Aspergillus flavus, and Sensory Quality in Fig Fruit, during Controlled Storage. Plants 2021, 10, 112. [Google Scholar] [CrossRef]
- Khaliq, G.; Muda Mohamed, M.T.; Ali, A.; Ding, P.; Ghazali, H.M. Effect of gum arabic coating combined with calcium chloride on physico-chemical and qualitative properties of mango (Mangifera indica L.) fruit during low temperature storage. Sci. Hortic. 2015, 190, 187–194. [Google Scholar] [CrossRef]
- Ahmad, S.; Thompson, A.K.; Asi, A.A.; Khan, M.; Chatha, G.A.; Shahid, M.A. Effect of reduced O2 and increased CO2 (controlled atmosphere storage) on the ripening and quality of ethylene treated banana fruit. Int. J. Agric. Biol. 2001, 3, 486–490. [Google Scholar]
- Ju, Z.; Duan, Y.; Ju, Z. Plant oil emulsion modifies internal atmosphere, delays fruit ripening, and inhibits internal browning in Chinese pears. Postharvest Biol. Technol. 2000, 20, 243–250. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y. Effects of chitosan coating on postharvest life and quality of longan fruit. Food Chem. 2001, 73, 139–143. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Singh, Z.; Khan, A.S. Postharvest Aloe vera gel-coating modulates fruit ripening and quality of ‘Arctic Snow’ nectarine kept in ambient and cold storage. Int. J. Food Sci. Technol. 2009, 44, 1024–1033. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate Coatings Preserve Fruit Quality and Bioactive Compounds during Storage of Sweet Cherry Fruit. Food Bioprocess Technol. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Guilbert, S.; Gontard, N.; Gorris, L.G.M. Prolongation of the Shelf-life of Perishable Food Products using Biodegradable Films and Coatings. LWT Food Sci. Technol. 1996, 29, 10–17. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Gavara, R.; Chiralt, A. Barrier properties of sodium caseinate films as affected by lipid composition and moisture content. J. Food Eng. 2012, 109, 372–379. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S. Application and Evaluation of a Pectin-Based Edible Coating Process for Quality Change Kinetics and Shelf-Life Extension of Lime Fruit (Citrus aurantifolium). Coatings 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality, 1st ed.; CRC-Taylor & Francis: Boca Raton, FL, USA, 2010; p. 288. [Google Scholar] [CrossRef]
- Chardonnet, C.O.; Charron, C.S.; Sams, C.E.; Conway, W.S. Chemical changes in the cortical tissue and cell walls of calcium-infiltrated ‘Golden Delicious’ apples during storage. Postharvest Biol. Technol. 2003, 28, 97–111. [Google Scholar] [CrossRef]
- Serrano, M.A.; Martínez-Romero, D.; Castillo, S.; Guillén, F.; Valero, D. Role of calcium and heat treatments in alleviating physiological changes induced by mechanical damage in plum. Postharvest Biol. Technol. 2004, 34, 155–167. [Google Scholar] [CrossRef]
- Verdini, R.A.; Zorrilla, S.E.; Rubiolo, A.C. Calcium Uptake during Immersion of Strawberries in CaCl2 Solutions. J. Food Sci. 2008, 73, C533–C539. [Google Scholar] [CrossRef]
- Ortiz, A.; Graell, J.; Lara, I. Cell wall-modifying enzymes and firmness loss in ripening ‘Golden Reinders’ apples: A comparison between calcium dips and ULO storage. Food Chem. 2011, 128, 1072–1079. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.B.; Sargent, S.A. Possible Influences of Water Loss and Polyphenol Oxidase Activity on Anthocyanin Content and Discoloration in Fresh Ripe Strawberry (cv. Oso Grande) during Storage at 1 °C. J. Food Sci. 2005, 70, S79–S84. [Google Scholar] [CrossRef]
- Alonso, J.; Alique, R. Sweet Cherries. In Handbook of Fruits and Fruit Processing; Blackwell Publishing: Ames, IA, USA, 2006; pp. 359–367. [Google Scholar] [CrossRef]
- Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan Coating to Preserve the Qualitative Traits and Improve Antioxidant System in Fresh Figs (Ficus carica L.). Agriculture 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]






| Sample | T (°C) | Cross Model Parameters | R2 | SDE | MRE | ||
|---|---|---|---|---|---|---|---|
| Ƞ0 (Pa·s) | λ (s) | m | |||||
| C-OO | 4 | 0.612 (0.003) | 0.003 (0.0001) | 0.711 (0.017) | 0.997 | 0.025 | 3.2 |
| 25 | 0.283 (0.004) | 0.001 (0.0001) | 0.609 (0.065) | 0.971 | 0.024 | 4.7 | |
| A-OO | 4 | 0.620 (0.002) | 0.010 (0.0002) | 0.717 (0.009) | 0.999 | 0.023 | 3.1 |
| 25 | 0.346 (0.004) | 0.004 (0.0003) | 0.781 (0.045) | 0.990 | 0.032 | 6.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, T.M.; Moldão-Martins, M.; Alves, V.D. Composite Coatings of Chitosan and Alginate Emulsions with Olive Oil to Enhance Postharvest Quality and Shelf Life of Fresh Figs (Ficus carica L. cv. ‘Pingo De Mel’). Foods 2021, 10, 718. https://doi.org/10.3390/foods10040718
Vieira TM, Moldão-Martins M, Alves VD. Composite Coatings of Chitosan and Alginate Emulsions with Olive Oil to Enhance Postharvest Quality and Shelf Life of Fresh Figs (Ficus carica L. cv. ‘Pingo De Mel’). Foods. 2021; 10(4):718. https://doi.org/10.3390/foods10040718
Chicago/Turabian StyleVieira, Tiago M., Margarida Moldão-Martins, and Vítor D. Alves. 2021. "Composite Coatings of Chitosan and Alginate Emulsions with Olive Oil to Enhance Postharvest Quality and Shelf Life of Fresh Figs (Ficus carica L. cv. ‘Pingo De Mel’)" Foods 10, no. 4: 718. https://doi.org/10.3390/foods10040718