Fabrication of Oil-in-Water Emulsions with Whey Protein Isolate–Puerarin Composites: Environmental Stability and Interfacial Behavior
Abstract
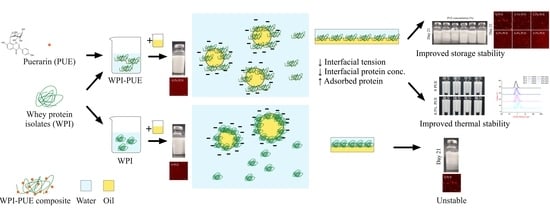
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Emulsions
2.3. Characterizations of the Emulsions
2.3.1. Particle Size
2.3.2. ζ-Potential
2.4. Confocal Laser Scanning Microscopy
2.5. Interfacial Protein Concentration
2.6. Dynamic Interfacial Properties
2.7. Physical Storage Stability
2.8. Influence of Environmental Conditions on Emulsion Stability
2.8.1. pH
2.8.2. Ionic Strength
2.8.3. Heat
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Oil-in-Water Emulsions Prepared Using WPI–PUE Composites
3.2. Storage Stability of Emulsions
3.3. Influence of Environmental Conditions on the Emulsions
3.3.1. Influence of pH on the Emulsion
3.3.2. Influence of Ionic Strength on the Emulsion
3.3.3. Influence of Thermal Treatment on the Emulsion
3.4. Adsorption of WPI–PUE Composites at the Oil–Water Interface
3.5. Interfacial Tension of WPI–PUE Composites at the Oil–Water Interface
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Yang, B.A.O.; Du, X. Investigation of supercritical fluid extraction of puerarin from pueraria lobata. J. Food Process Eng. 2009, 32, 682–691. [Google Scholar] [CrossRef]
- Jin, S.E.; Son, Y.K.; Min, B.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory and antioxidant activities of constituents isolated from Pueraria lobata roots. Arch. Pharm. Res. 2012, 35, 823–837. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhao, J.; Dai, T.; McClements, D.J.; Liu, C. The effect of whey protein-puerarin interactions on the formation and performance of protein hydrogels. Food Hydrocoll. 2020, 113, 106444. [Google Scholar] [CrossRef]
- Chen, E.; Cao, L.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Enhancement of physicochemical properties of whey protein-stabilized nanoemulsions by interfacial cross-linking using cinnamaldehyde. Food Hydrocoll. 2018, 77, 976–985. [Google Scholar] [CrossRef]
- Monahan, F.J.; German, J.B.; Kinsella, J.E. Effect of pH and temperature on protein unfolding and thiol/disulfide interchange reactions during heat-induced gelation of whey proteins. J. Agric. Food Chem. 1995, 43, 46–52. [Google Scholar] [CrossRef]
- Tian, L.; Kejing, Y.; Zhang, S.; Yi, J.; Zhu, Z.; Decker, E.A.; McClements, D.J. Impact of tea polyphenols on the stability of oil-in-water emulsions coated by whey proteins. Food Chem. 2021, 343, 128448. [Google Scholar] [CrossRef] [PubMed]
- Demetriades, K.; Coupland, J.N.; McClements, D.J. Physical properties of whey protein stabilized emulsions as related to ph and nacl. J. Food Sci. 1997, 62, 342–347. [Google Scholar] [CrossRef]
- Guo, B.; Hu, X.; Wu, J.; Chen, R.; Dai, T.; Liu, Y.; Luo, S.; Liu, C. Soluble starch/whey protein isolate complex-stabilized high internal phase emulsion: Interaction and stability. Food Hydrocoll. 2021, 111, 106377. [Google Scholar] [CrossRef]
- Setiowati, A.D.; Wijaya, W.; Van der Meeren, P. Whey protein-polysaccharide conjugates obtained via dry heat treatment to improve the heat stability of whey protein stabilized emulsions. Trends Food Sci. Technol. 2020, 98, 150–161. [Google Scholar] [CrossRef]
- Gülseren, İ.; Fang, Y.; Corredig, M. Whey protein nanoparticles prepared with desolvation with ethanol: Characterization, thermal stability and interfacial behavior. Food Hydrocoll. 2012, 29, 258–264. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Plundrich, N.; Schneider, M.; Campbell, C.; Lila, M.A. Protein-polyphenol particles for delivering structural and health functionality. Food Hydrocoll. 2017, 72, 163–173. [Google Scholar] [CrossRef]
- Li, R.; Dai, T.; Tan, Y.; Fu, G.; Wan, Y.; Liu, C.; McClements, D.J. Fabrication of pea protein-tannic acid complexes: Impact on formation, stability, and digestion of flaxseed oil emulsions. Food Chem. 2020, 310, 125828. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wan, J.; You, M.; McClements, D.J.; Li, Y.; Li, B. Impact of whey protein complexation with phytic acid on its emulsification and stabilization properties. Food Hydrocoll. 2019, 87, 90–96. [Google Scholar] [CrossRef]
- Dai, T.; Li, R.; Liu, C.; Liu, W.; Li, T.; Chen, J.; Kharat, M.; McClements, D.J. Effect of rice glutelin-resveratrol interactions on the formation and stability of emulsions: A multiphotonic spectroscopy and molecular docking study. Food Hydrocoll. 2019, 97, 105234. [Google Scholar] [CrossRef]
- Pham, L.B.; Wang, B.; Zisu, B.; Adhikari, B. Complexation between flaxseed protein isolate and phenolic compounds: Effects on interfacial, emulsifying and antioxidant properties of emulsions. Food Hydrocoll. 2019, 94, 20–29. [Google Scholar] [CrossRef]
- Dai, T.; Chen, J.; McClements, D.J.; Hu, P.; Ye, X.; Liu, C.; Li, T. Protein-polyphenol interactions enhance the antioxidant capacity of phenolics: Analysis of rice glutelin-procyanidin dimer interactions. Food Funct. 2019, 10, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Li, T.; Li, R.; Zhou, H.; Liu, C.; Chen, J.; McClements, D.J. Utilization of plant-based protein-polyphenol complexes to form and stabilize emulsions: Pea proteins and grape seed proanthocyanidins. Food Chem. 2020, 329, 127219. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; McClements, D.J.; Zhu, Y.; Zou, L.; Zhou, W.; Liu, W. Fabrication of osa starch/chitosan polysaccharide-based high internal phase emulsion via altering interfacial behaviors. J. Agric. Food Chem. 2019, 67, 10937–10946. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiang, X.; Wang, X.; Zhang, Y.; Hu, M.; Chen, T.; Liu, C. Fabrication and characterization of oil-in-water emulsions stabilized by macadamia protein isolate/chitosan hydrochloride composite polymers. Food Hydrocoll. 2020, 103, 105655. [Google Scholar] [CrossRef]
- Guo, B.; Hu, X.; Deng, F.; Wu, J.; Luo, S.; Chen, R.; Liu, C. Supernatant starch fraction of corn starch and its emulsifying ability: Effect of the amylose content. Food Hydrocoll. 2020, 103, 105711. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Zhu, Y.; Chen, Y.; Zou, L.; Liu, W.; Cheng, C.; Fu, D.; Liu, C. Enhancement of the solubility, stability and bioaccessibility of quercetin using protein-based excipient emulsions. Food Res. Int. 2018, 114, 30–37. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Z.; Wang, Y.; Chen, J.; Zhong, Y.; Liang, R.; Peng, S.; McClements, D.J.; Liu, W. Tunable high internal phase emulsions (HIPEs) formulated using lactoferrin-gum Arabic complexes. Food Hydrocoll. 2021, 113, 106445. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, Q.; Liu, X.; Xu, Y.; Yang, L.; Wang, S.; Li, J.; He, Y.; Liu, H. Soy glycinin-soyasaponin mixtures at oil-water interface: Interfacial behavior and O/W emulsion stability. Food Chem. 2020, 327, 127062. [Google Scholar] [CrossRef] [PubMed]
- Seczyk, L.; Swieca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein-phenolic interactions as a factor affecting the physicochemical properties of white bean proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Lin, Z.; Zhou, J.; Yun, G.; Guo, R.; Richardson, J.J.; Caruso, F. Polyphenol-mediated assembly of proteins for engineering functional materials. Angew. Chem. Int. Ed. Engl. 2020, 59, 15618–15625. [Google Scholar] [CrossRef]
- Freer, E.M.; Yim, K.S.; Fuller, G.G.; Radke, C.J. Interfacial rheology of globular and flexible proteins at the hexadecane/water interface: Comparison of shear and dilatation deformation. J. Phys. Chem. B 2004, 108, 3835–3844. [Google Scholar] [CrossRef]
- McClements, D.J.; Monahan, F.J.; Kinsella, J.E. Disulfide bond formation affects stability of whey protein isolate emulsions. J. Food Sci. 1993, 58, 1036–1039. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, F.; Xie, B.; Sun, Z.; McClements, D.J.; Deng, Q. Fabrication and characterization of whey protein isolates- lotus seedpod proanthocyanin conjugate: Its potential application in oxidizable emulsions. Food Chem. 2021, 346, 128680. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.A.; Dalgleish, D.G. Effect of pH on the stability and surface composition of emulsions made with whey protein isolate. J. Agric. Food Chem. 1994, 42, 2131–2135. [Google Scholar] [CrossRef]
- Ye, A.; Singh, H. Heat stability of oil-in-water emulsions formed with intact or hydrolysed whey proteins: Influence of polysaccharides. Food Hydrocoll. 2006, 20, 269–276. [Google Scholar] [CrossRef]
- Dickinson, E.; Parkinson, E.L. Heat-induced aggregation of milk protein-stabilized emulsions: Sensitivity to processing and composition. Int. Dairy J. 2004, 14, 635–645. [Google Scholar] [CrossRef]
- Seta, L.; Baldino, N.; Gabriele, D.; Lupi, F.R.; Cindio, B.d. Rheology and adsorption behaviour of β-casein and β-lactoglobulin mixed layers at the sunflower oil/water interface. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 669–677. [Google Scholar] [CrossRef]
- Hu, J.-N.; Zheng, H.; Chen, X.-X.; Li, X.; Xu, Y.; Xu, M.-F. Synergetic effects of whey protein isolate and naringin on physical and oxidative stability of oil-in-water emulsions. Food Hydrocoll. 2020, 101, 105517. [Google Scholar] [CrossRef]
- Von Staszewski, M.; Pizones Ruiz-Henestrosa, V.M.; Pilosof, A.M.R. Green tea polyphenols-β-lactoglobulin nanocomplexes: Interfacial behavior, emulsification and oxidation stability of fish oil. Food Hydrocoll. 2014, 35, 505–511. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Zhao, J.; Dai, T.; Ye, J.; Wu, J.; Chen, T.; Liu, C. Fabrication of Oil-in-Water Emulsions with Whey Protein Isolate–Puerarin Composites: Environmental Stability and Interfacial Behavior. Foods 2021, 10, 705. https://doi.org/10.3390/foods10040705
Zhong Y, Zhao J, Dai T, Ye J, Wu J, Chen T, Liu C. Fabrication of Oil-in-Water Emulsions with Whey Protein Isolate–Puerarin Composites: Environmental Stability and Interfacial Behavior. Foods. 2021; 10(4):705. https://doi.org/10.3390/foods10040705
Chicago/Turabian StyleZhong, Yejun, Jincheng Zhao, Taotao Dai, Jiangping Ye, Jianyong Wu, Tingting Chen, and Chengmei Liu. 2021. "Fabrication of Oil-in-Water Emulsions with Whey Protein Isolate–Puerarin Composites: Environmental Stability and Interfacial Behavior" Foods 10, no. 4: 705. https://doi.org/10.3390/foods10040705