Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review
Abstract
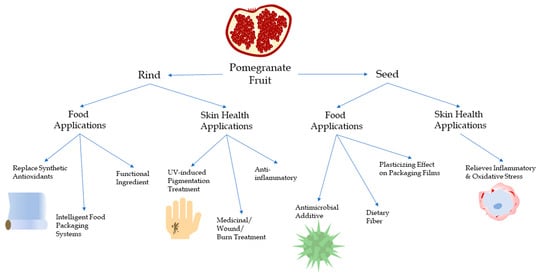
:1. Introduction
1.1. Properties and Chemical Composition of Pomegranate
1.2. Pomegranate Seed
1.3. Pomegranate Rind
2. Food Applications
2.1. Food Additives
2.1.1. Antioxidant and Antimicrobial
2.1.2. Pectin
2.1.3. Fiber
2.2. Food Packaging/Bioplastics
2.2.1. Active Ingredient with Antioxidant and Antimicrobial Effects
2.2.2. Packaging Color
2.2.3. Plasticizing, Strengthening, and Elongation Effects
3. Skin Health Applications
3.1. Skin Whitening
3.2. Skin Wrinkling and Skin Aging
3.3. Burn and Wound Healing
3.4. Anti-Inflammatory and Anti-Pain
3.5. Anti-Bacterial and Anti-Fungal
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- America’s Food Waste Problem. 2016. Available online: https://www.epa.gov/sciencematters/americas-food-waste-problem (accessed on 22 April 2016).
- Facts and Figures About Materials, Waste and Recycling. 2018. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/food-material-specific-data (accessed on 12 December 2020).
- Lieber, C. Vox. A Scientist on the Myth of Ugly Produce and Food Waste. 2019. Available online: https://www.vox.com/the-goods/2019/2/26/18240399/food-waste-ugly-produce-myths-farms (accessed on 26 February 2019).
- Marzolo, G. Pomegranates. 2015. Available online: https://www.agmrc.org/commodities-products/fruits/pomegranates (accessed on 12 August 2015).
- Fruit & Nut Research & Information Center. Pomegranate. Available online: http://fruitandnuteducation.ucdavis.edu/fruitnutproduction/Pomegranate/ (accessed on 13 January 2019).
- California Department of Food and Agriculture. California County Agricultural Commissioners’ Reports Crop: Year 2017–2018; California Department of Food and Agriculture: Sacramento, CA, USA, 2018; p. 95.
- Day, K.R.; Andris, H.L.; Klonsky, K.M.; De Moura, R.L. Sample Costs to Establish and Produce Pomegranates; University of California Cooperative Extension: Napa, CA, USA, 2010; pp. 1–20. [Google Scholar]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef]
- Wikandari, R.; Nguyen, H.; Millati, R.; Niklasson, C.; Taherzadeh, M.J. Improvement of Biogas Production from Orange Peel Waste by Leaching of Limonene. BioMed Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Charalampia, D.; Koutelidakis, A. From Pomegranate Processing By-Products to Innovative value added Func-tional Ingredients and Bio-Based Products with Several Applications in Food Sector. BAOJ Biotech. 2017, 3, 210. [Google Scholar]
- Verardo, V.; Garcia-Salas, P.; Baldi, E.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Pomegranate seeds as a source of nutraceutical oil naturally rich in bioactive lipids. Food Res. Int. 2014, 65, 445–452. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compreh. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, P.C.; Kirtane, R.D.; Chaudhari, A.B.; Kothari, R.M. Conservation and recycling of pomegranate seeds and shells for value addition. J. Renew. Sustain. Energy 2009, 1, 013107. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Białek, A.; Białek, M.; Lepionka, T.; Tober, E.; Czauderna, M. The Quality Determination of Selected Commercial Online Purchased Edible Pomegranate Seed Oils With New Argentometric Liquid Chromatography Method. J. Diet. Suppl. 2020, 1–21. [Google Scholar] [CrossRef]
- Khajebishak, Y.; Payahoo, L.; Alivand, M.; Alipour, B. Punicic acid: A potential compound of pomegranate seed oil in Type 2 diabetes mellitus management. J. Cell. Physiol. 2019, 234, 2112–2120. [Google Scholar] [CrossRef]
- Hora, J.J.; Maydew, E.R.; Lansky, E.P.; Dwivedi, C. Chemopreventive Effects of Pomegranate Seed Oil on Skin Tumor Development in CD1 Mice. J. Med. Food 2003, 6, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Nekooeian, A.A.; Eftekhari, M.H.; Adibi, S.; Rajaeifard, A. Effects of Pomegranate Seed Oil on Insulin Release in Rats with Type 2 Diabetes. Iran. J. Med. Sci. 2014, 39, 130–135. [Google Scholar]
- Aruna, P.; Venkataramanamma, D.; Singh, A.K.; Singh, R. Health Benefits of Punicic Acid: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 15, 16–27. [Google Scholar] [CrossRef]
- Khoddami, A.; Man, Y.B.C.; Roberts, T.H. Physico-chemical properties and fatty acid profile of seed oils from pomegranate (Punica granatum L.) extracted by cold pressing. Eur. J. Lipid Sci. Technol. 2014, 116, 553–562. [Google Scholar] [CrossRef]
- Gül, H.; Şen, H. Effects of pomegranate seed flour on dough rheology and bread quality. CyTA J. Food 2017, 15, 622–628. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, S.; Bhat, Z.F. Utilization of pomegranate seed powder and tomato powder in the development of fi-ber-enriched chicken nuggets. Nutr. Food Sci. 2015, 45, 793–807. [Google Scholar] [CrossRef]
- Abbasi, H.; Rezaei, K.; Emamdjomeh, Z.; Mousavi, S.M.E. Effect of various extraction conditions on the phenolic contents of pomegranate seed oil. Eur. J. Lipid Sci. Technol. 2008, 110, 435–440. [Google Scholar] [CrossRef]
- Ahangari, B.; Sargolzaei, J. Extraction of pomegranate seed oil using subcritical propane and supercritical carbon dioxide. Theor. Found. Chem. Eng. 2012, 46, 258–265. [Google Scholar] [CrossRef]
- Ververi, M.; Goula, A.M. Pomegranate peel and orange juice by-product as new biosorbents of phenolic compounds from olive mill wastewaters. Chem. Eng. Process. Process. Intensif. 2019, 138, 86–96. [Google Scholar] [CrossRef]
- Amin, N.K. Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2009, 165, 52–62. [Google Scholar] [CrossRef]
- Saadi, W.; Rodríguez-Sánchez, S.; Ruiz, B.; Souissi-Najar, S.; Ouederni, A.; Fuente, E. Pyrolysis technologies for pomegranate (Punica granatum L.) peel wastes. Prospects in the bioenergy sector. Renew. Energy 2019, 136, 373–382. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; VijayRaghavan, R.; Arora, A. Complete Utilization of Waste Pomegranate Peels to Produce a Hydrocolloid, Punicalagin Rich Phenolics, and a Hard Carbon Electrode. ACS Sustain. Chem. Eng. 2018, 6, 16363–16374. [Google Scholar] [CrossRef]
- Hossin, F.L.A. Effect of Pomegranate (Punica granatum) Peels and It’s Extract on Obese Hypercholesterolemic Rats. Pak. J. Nutr. 2009, 8, 1251–1257. [Google Scholar] [CrossRef] [Green Version]
- Ismail, T.; Akhtar, S.; Riaz, M.; Ismail, A. Effect of pomegranate peel supplementation on nutritional, organoleptic and stability properties of cookies. Int. J. Food Sci. Nutr. 2014, 65, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. In vitro bioaccessibility and functional properties of polyphenols from pom-egranate peels and pomegranate peels-enriched cookies. Food Funct. 2016, 7, 4247–4258. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Yaich, H.; Hidouri, H.; Attia, H.; Ayadi, M. Effect of substituted gelling agents from pomegranate peel on colour, textural and sensory properties of pomegranate jam. Food Chem. 2018, 239, 1047–1054. [Google Scholar] [CrossRef]
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M.; Ghafoor, K. Characterization of pomegranate (Punica granatum L.) seed and oils. Eur. J. Lipid Sci. Technol. 2017, 119, 1700074. [Google Scholar] [CrossRef]
- Abid, M.; Cheikhrouhou, S.; Renard, C.M.; Bureau, S.; Cuvelier, G.; Attia, H.; Ayadi, M. Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 2017, 215, 318–325. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M.; Adamopoulos, K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 2015, 31, 204–215. [Google Scholar] [CrossRef]
- Elfalleh, W. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 2012, 6, 4724–4730. [Google Scholar] [CrossRef]
- Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review. Processes 2021, 9, 236. [Google Scholar] [CrossRef]
- Haidari, M.; Ali, M.; Casscells, S.W.; Madjid, M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine 2009, 16, 1127–1136. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Shabtay, A.; Eitam, H.; Tadmor, Y.; Orlov, A.; Meir, A.; Weinberg, P.; Weinberg, Z.G.; Chen, Y.; Brosh, A.; Izhaki, I.; et al. Nutritive and Antioxidative Potential of Fresh and Stored Pomegranate Industrial Byproduct as a Novel Beef Cattle Feed. J. Agric. Food Chem. 2008, 56, 10063–10070. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.; Golian, A.; Kermanshahi, H.; Mirakzehi, M.T. Effects of dietary α-tocopherol acetate, pomegranate peel, and pomegranate peel extract on phenolic content, fatty acid composition, and meat quality of broiler chickens. J. Appl. Anim. Res. 2016, 45, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Yaseen, A.T.; El-Kholy, M.E.-S.H.; El-Razik, W.M.A.; Soliman, M.H. Effect of Using Pomegranate Peel Extract as Feed Additive on Performance, Serum Lipids and Immunity of Broiler Chicks. Zagazig Vet. J. 2014, 42, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Sharifian, M.; Hosseini-Vashan, S.; Nasri, M.F.; Perai, A. Pomegranate peel extract for broiler chickens under heat stress: Its influence on growth performance, carcass traits, blood metabolites, immunity, jejunal morphology, and meat quality. Livest. Sci. 2019, 227, 22–28. [Google Scholar] [CrossRef]
- Acar, Ü.; Parrino, V.; Kesbiç, O.S.; Paro, G.L.; Saoca, C.; Abbate, F.; Yılmaz, S.; Fazio, F. Effects of Different Levels of Pomegranate Seed Oil on Some Blood Parameters and Disease Resistance Against Yersinia ruckeri in Rainbow Trout. Front. Physiol. 2018, 9, 596. [Google Scholar] [CrossRef]
- Tabaraki, R.; Heidarizadi, E.; Benvidi, A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep. Purif. Technol. 2012, 98, 16–23. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Shahamirian, M.; Eskandari, M.H.; Niakousari, M.; Esteghlal, S.; Gahruie, H.H.; Khaneghah, A.M. Incorporation of pomegranate rind powder extract and pomegranate juice into frozen burgers: Oxi-dative stability, sensorial and microbiological characteristics. J. Food Sci. Technol. 2019, 56, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, D.; Bhat, Z.; Kumar, S. Pomegranate (Punica granatum) rind extract as a novel preservative in cheese. Food Biosci. 2015, 12, 47–53. [Google Scholar] [CrossRef]
- Ali, M.N.; Prasad, S.G.; Singh, M. Functional, Antioxidant and Sensory Qualities of Ice-Cream from Pomegranate Seed Powder. Asian J. Chem. 2016, 28, 2013–2016. [Google Scholar] [CrossRef]
- Çam, M.; İçyer, N.C.; Erdoğan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Colantuono, A.; Vitaglione, P.; Ferracane, R.; Campanella, O.H.; Hamaker, B.R. Development and functional characterization of new antioxidant dietary fibers from pomegranate, olive and artichoke by-products. Food Res. Int. 2017, 101, 155–164. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M. Encapsulation of pomegranate peel extract with a new carrier material from orange juice by-products. J. Food Eng. 2019, 253, 1–13. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Pectins. Food & Beverage/North America/Hydrocolloids. Available online: https://www.cargill.com/food-bev/emea/hydrocolloids/pectin (accessed on 11 November 1998).
- Staff, M.C. Dietary Fiber: Essential for a Healthy Diet. Healthy Lifestyle. 2018. Available online: https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/fiber/art-20043983 (accessed on 6 January 2021).
- Pamisetty, A.; Kumar, K.A.; Indrani, D.; Singh, R.P. Rheological, physico-sensory and antioxidant properties of punicic acid rich wheat bread. J. Food Sci. Technol. 2020, 57, 253–262. [Google Scholar] [CrossRef]
- Bertolo, M.R.; Martins, V.C.; Horn, M.M.; Brenelli, L.B.; Plepis, A.M. Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pome-granate peel extract. Carbohydr. Polym. 2020, 228, 115386. [Google Scholar] [CrossRef]
- Hanani, Z.N.; Yee, F.C.; Nor-Khaizura, M.A. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Cui, H.; Surendhiran, D.; Li, C.; Lin, L. Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf Life 2020, 24, 100511. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 2016, 59, 818–823. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of Nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Licciardello, F.; Kharchoufi, S.; Muratore, G.; Restuccia, C. Effect of edible coating combined with pomegranate peel extract on the quality maintenance of white shrimps (Parapenaeus longirostris) during refrigerated storage. Food Packag. Shelf Life 2018, 17, 114–119. [Google Scholar] [CrossRef]
- Oliveira, T.; Ítalo, S.; Zea-Redondo, L.; Moates, G.K.; Wellner, N.; Cross, K.; Waldron, K.W.; Azeredo, H.M. Pomegranate peel pectin films as affected by montmorillonite. Food Chem. 2016, 198, 107–112. [Google Scholar] [CrossRef]
- Sogut, E.; Balqis, A.I.; Hanani, Z.N.; Seydim, A.C. The properties of κ-carrageenan and whey protein isolate blended films containing pomegranate seed oil. Polym. Test. 2019, 77, 105886. [Google Scholar] [CrossRef]
- Teodosio, A.E.; Araujo, R.H.; Santos, B.G.; Linne, J.A.; Silva, K.G.; Gomes, F.A.; Souza, G.L.; Lima, J.F. Analysis of bioactive compounds in umbu (Spondias tuberosa) by application of edible coating based on Chlorella sp during storage. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, M.; Watanabe, Y.; Kasai, K.; Yamakoshi, J.; Koga, T. Inhibitory Effect of an Ellagic Acid-Rich Pomegranate Extract on Tyrosinase Activity and Ultravio-let-Induced Pigmentation. Biosci. Biotechnol. Biochem. 2005, 69, 2368–2373. [Google Scholar] [CrossRef]
- Houston, D.M.; Bugert, J.; Denyer, S.P.; Heard, C.M. Anti-inflammatory activity of Punica granatum L. (Pomegranate) rind extracts applied topically to ex vivo skin. Eur. J. Pharm. Biopharm. 2017, 112, 30–37. [Google Scholar] [CrossRef]
- Foss, S.R.; Nakamura, C.V.; Ueda-Nakamura, T.; Cortez, D.A.G.; Endo, E.H.; Filho, B.P.D. Anti-fungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 32. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Chongnativisit, W.; Chaikul, P.; Lourith, N. Phenolic-rich Pomegranate Peel Extract: In Vitro, Cellular, and In Vivo Activities for Skin Hy-perpigmentation Treatment. Planta Med. 2020, 86, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Nakatani, S.; Onodera, H.; Nagatomo, A.; Nishida, N.; Matsuura, Y.; Kobata, K.; Wada, M. Anti-Glycation Effects of Pomegranate (Punica granatum L.) Fruit Extract and Its Components in Vivo and in Vitro. J. Agric. Food Chem. 2015, 63, 7760–7764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirsane, S.; Mirsane, S. Benefits of ellagic acid from grapes and pomegranates against colorectal cancer. Caspian J. Intern. Med. 2017, 8, 226–227. [Google Scholar] [PubMed]
- Turrini, F.; Malaspina, P.; Giordani, P.; Catena, S.; Zunin, P.; Boggia, R. Traditional Decoction and PUAE Aqueous Extracts of Pomegranate Peels as Potential Low-Cost An-ti-Tyrosinase Ingredients. Appl. Sci. 2020, 10, 2795. [Google Scholar] [CrossRef] [Green Version]
- Bogdan, C.; Iurian, S.; Tomuta, I.; Moldovan, M.L. Improvement of skin condition in striae distensae: Development, characterization and clinical efficacy of a cosmetic product containing Punica granatum seed oil and Croton lechleri resin extract. Drug Des. Dev. Ther. 2017, 11, 521–531. [Google Scholar] [CrossRef] [Green Version]
- Baltazar, D.; Marto, J.; Berger, T.; Pinto, P.; Ribeiro, H.M. The antiageing efficacy of donkey milk in combination with pomegranate extract and UV protection: A traditional ingredient in a modern formulation. Monogr. Spec. Issue Cosmet. Act. Ingred. H&PC Today Househ. Pers. Care Today 2017, 12, 30–32. [Google Scholar]
- Bae, J.-Y.; Choi, J.-S.; Kang, S.-W.; Lee, Y.-J.; Park, J.; Kang, Y.-H. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp. Dermatol. 2010, 19, e182–e190. [Google Scholar] [CrossRef]
- Rout, S.; Banerjee, R. Free radical scavenging, anti-glycation and tyrosinase inhibition properties of a polysaccharide frac-tion isolated from the rind from Punica granatum. Bioresour. Technol. 2007, 98, 3159–3163. [Google Scholar] [CrossRef]
- Yagi, M.; Parengkuan, L.; Sugimura, H.; Shioya, N.; Matsuura, Y.; Nishida, N.; Nagatomo, A.; Yonei, Y. Anti-glycation effect of pomegranate (Punica granatum L.) extract: An open clinical study. Glycative Stress Res. 2014, 1, 060–067. [Google Scholar]
- Castiel, I.; Gueniche, A. Non-Therapeutic Cosmetic Use of at Least One Pomegranate Extract, as Agent for Firming Skin of a Subject, Who Has Weight Modification Prior to and/or After an Aesthetic Surgery and to Prevent and/or Treat Sagging Skin, in Espcenet. FR2967063A1, 11 May 2012. [Google Scholar]
- Wilson, K. Lip Gloss. US 2012/0288319 A1, 15 November 2012. [Google Scholar]
- Kasai, K.; Yoshimura, M.; Koga, T.; Arii, M.; Kawasaki, S. Effects of oral administration of ellagic acid-rich pomegranate extract on ultraviolet-induced pigmentation in the human skin. J. Nutr. Sci. Vitaminol. 2006, 52, 383–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukiswanto, B.S.; Miranti, A.; Sudjarwo, S.A.; Primarizky, H.; Yuniarti, W.M. Evaluation of wound healing potential of pomegranate (Punica granatum) whole fruit extract on skin burn wound in rats (Rattus norvegicus). J. Adv. Vet. Anim. Res. 2019, 6, 202–207. [Google Scholar] [CrossRef]
- Yuniarti, W.M.; Primarizky, H.; Lukiswanto, B.S. The activity of pomegranate extract standardized 40% ellagic acid during the healing process of incision wounds in albino rats (Rattus norvegicus). Vet. World 2018, 11, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Nitiruangjaras, A.; Reanmongkol, W. Wound healing activities of standardized pomegranate rind extract and its major antioxidant ellagic acid in rat dermal wounds. J. Nat. Med. 2014, 68, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.M.; Robins, B.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. In vitro permeation and biological activity of punicalagin and zinc (II) across skin and mucous membranes prone to Herpes simplex virus infection. Eur. J. Pharm. Sci. 2017, 96, 99–106. [Google Scholar] [CrossRef]
- Houston, D.M.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV. PLoS ONE 2017, 12, e0179291. [Google Scholar]
- Marchiori, M.C.L.; Rigon, C.; Camponogara, C.; Oliveira, S.M.; Cruz, L. Hydrogel containing silibinin-loaded pomegranate oil based nanocapsules exhibits anti-inflammatory effects on skin damage UVB radiation-induced in mice. J. Photochem. Photobiol. B Biol. 2017, 170, 25–32. [Google Scholar] [CrossRef]
- Baccarin, T.; Mitjans, M.; Ramos, D.; Lemos-Senna, E.; Vinardell, M.P. Photoprotection by Punica granatum seed oil nanoemulsion entrapping polyphenol-rich ethyl acetate fraction against UVB-induced DNA damage in human keratinocyte (HaCaT) cell line. J. Photochem. Photobiol. B Biol. 2015, 153, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Guo, H.; DaSilva, N.A.; Li, D.; Zhang, K.; Wan, Y.; Gao, X.H.; Chen, H.D.; Seeram, N.P.; Ma, H. Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide-induced oxidative stress and cyto-toxicity in human keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef]
- Zekry, S.S.A.; Abdellatif, A.; Azzazy, H.M. Fabrication of pomegranate/honey nanofibers for use as anti-bacterial wound dressings. Wound Med. 2020, 28, 100181. [Google Scholar] [CrossRef]
- Tito, A.; Colantuono, A.; Pirone, L.; Pedone, E.M.; Intartaglia, D.; Giamundo, G.; Conte, I.; Vitaglione, P.; Apone, F. A pomegranate peel extract as inhibitor of SARS-CoV-2 Spike binding to human ACE2 (in vitro): A promising source of novel antiviral drugs. bioRxiv 2020. [Google Scholar] [CrossRef]
- Karimi, A.; Moradi, M.-T.; Rabiei, M.; Alidadi, S. In vitro anti-adenoviral activities of ethanol extract, fractions, and main phenolic compounds of pomegranate (Punica granatum L.) peel. Antivir. Chem. Chemother. 2020, 28, 2040206620916571. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, L.; Ascacio, A.; Rodriguez-Herrera, R.; Aguilera-Carbo, A.; Aguilar, C.N. ChemInform Abstract: Ellagic Acid: Biological Properties and Biotechnological Development for Production Processes. Afr. J. Biotechnol. 2012, 43, 4518–4523. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, Z.; Li, X.; Zhang, J.; Hu, X. Analysis of ellagic acid in pomegranate rinds by capillary electrophoresis and high-performance liquid chro-matography. Phytochem. Anal. 2008, 19, 86–89. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]


| Property | Seed Amount | Rind Amount |
|---|---|---|
| Moisture | 10.44–12.86% | 67.26–73.23% |
| Sugar | N/A | 30.65–34.83% |
| Crude Oil | 10.89–13.24% | N/A |
| Crude Protein | 6.71–8.11% | 3.96–7.13% |
| Crude Ash | 1.61–2.29% | 3.71–4.97% |
| Fiber | 17.33–27.84% | 28.10–33.93% |
| Pectin | N/A | 6.8–10.1% |
| Total Polyphenol (mg/g * GAE **) | Total Flavonoid (mg/g RE ***) | Total Anthocyanin (mg/g CGE ****) | Hydrolyzable Tannins (mg/g TAE *****) | |||||
|---|---|---|---|---|---|---|---|---|
| Extraction Method | Aq. | MeOH | Aq. | MeOH | Aq. | MeOH | Aq. | MeOH |
| Rind | 53.65 ± 4.13 | 85.60 ± 4.87 | 21.03 ± 1.62 | 51.52 ± 8.14 | 51.02 ± 10.33 | 102.20 ± 16.42 | 62.71 ± 11.32 | 139.63 ± 4.25 |
| Seed | 7.94 ± 1.25 | 11.84 ± 1.92 | 3.30 ± 0.52 | 6.79 ± 0.57 | 19.62 ± 3.12 | 40.84 ± 7.77 | 32.86 ± 4.24 | 29.57 ± 4.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. https://doi.org/10.3390/foods10030657
Ko K, Dadmohammadi Y, Abbaspourrad A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods. 2021; 10(3):657. https://doi.org/10.3390/foods10030657
Chicago/Turabian StyleKo, Katharine, Younas Dadmohammadi, and Alireza Abbaspourrad. 2021. "Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review" Foods 10, no. 3: 657. https://doi.org/10.3390/foods10030657