Antioxidant HPTLC-DPPH Fingerprinting of Honeys and Tracking of Antioxidant Constituents upon Thermal Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.2.1. Standard Solution and Reagent Preparations
2.2.2. Honey Sample Preparation
2.3. Chromatography
2.3.1. Sample Application
2.3.2. Development
2.3.3. Derivatisations
2.3.4. Data Analysis and Statistics
2.4. Validation
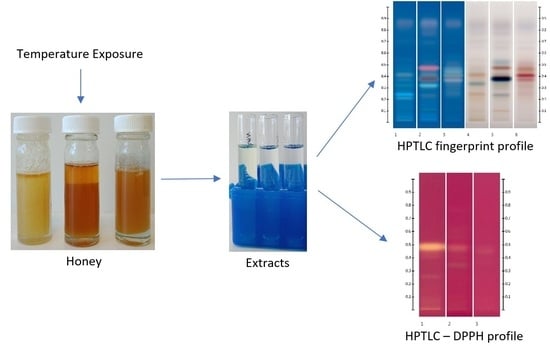
2.5. Thermal Exposure
3. Results and Discussion
3.1. Antioxidant Band Activity of Honeys
3.2. Comparison of Honey Extracts’ Floral Fingerprints with Antioxidant Fingerprints
3.3. Tracking of Thermal Stability of Selected Honeys
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Farsi, M.; Al-Amri, A.; Al-Hadhrami, A.; Al-Belushi, S. Color, flavonoids, phenolics and antioxidants of Omani honey. Heliyon 2018, 4, e00874. [Google Scholar] [CrossRef] [Green Version]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; Silva, W.P.d.; Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT-Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Alimentarius, C. Revised codex standard for honey. Codex Stand. 2001, 12, 1982. [Google Scholar]
- Soares, S.; Pinto, D.; Rodrigues, F.; Alves, R.C.; Oliveira, M. Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity. Molecules 2017, 22, 1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuropatnicki, A.K.; Kłósek, M.; Kucharzewski, M. Honey as medicine: Historical perspectives. J. Apic. Res. 2018, 57, 113–118. [Google Scholar] [CrossRef]
- Forrest, R.D. Early history of wound treatment. J. R. Soc. Med. 1982, 75, 198–205. [Google Scholar] [PubMed]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, N.; Engeseth, N.J. Antioxidant Capacity of Honeys from Various Floral Sources Based on the Determination of Oxygen Radical Absorbance Capacity and Inhibition of in Vitro Lipoprotein Oxidation in Human Serum Samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef] [PubMed]
- Masalha, M.; Abu-Lafi, S.; Abu-Farich, B.; Rayan, M.; Issa, N.; Zeidan, M.; Rayan, A. A New Approach for Indexing Honey for Its Heath/Medicinal Benefits: Visualization of the Concept by Indexing Based on Antioxidant and Antibacterial Activities. Medicines 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Haskoury, R.; Al-Waili, N.; Kamoun, Z.; Makni, M.; Al-Waili, H.; Lyoussi, B. Antioxidant Activity and Protective Effect of Carob Honey in CCl4-induced Kidney and Liver Injury. Arch. Med. Res. 2018, 49, 306–313. [Google Scholar] [CrossRef]
- Dzugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkok, D.; Silici, S. Antioxidant activities of honeybee products and their mixtures. Food Sci. Biotechnol. 2017, 26, 201–206. [Google Scholar] [CrossRef]
- Neupane, B.P.; Chaudhary, D.; Paudel, S.; Timsina, S.; Chapagain, B.; Jamarkattel, N.; Tiwari, B.R. Himalayan honey loaded iron oxide nanoparticles: Synthesis, characterization and study of antioxidant and antimicrobial activities. Int. J. Nanomed. 2019, 14, 3533–3541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marco, G.; Gismondi, A.; Panzanella, L.; Canuti, L.; Impei, S.; Leonardi, D.; Canini, A. Botanical influence on phenolic profile and antioxidant level of Italian honeys. J. Food Sci. Technol. 2018, 55, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
- Ronsisvalle, S.; Lissandrello, E.; Fuochi, V.; Petronio Petronio, G.; Straquadanio, C.; Crasci, L.; Panico, A.; Milito, M.; Cova, A.M.; Tempera, G.; et al. Antioxidant and antimicrobial properties of Casteanea sativa Miller chestnut honey produced on Mount Etna (Sicily). Nat. Prod. Res. 2019, 33, 843–850. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Otmani, I.; Abdennour, C.; Dridi, A.; Kahalerras, L.; Halima-Salem, A. Characteristics of the bitter and sweet honey from Algeria Mediterranean coast. Vet. World 2019, 12, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molaveisi, M.; Beigbabaei, A.; Akbari, E.; Noghabi, M.S.; Mohamadi, M. Kinetics of temperature effect on antioxidant activity, phenolic compounds and color of Iranian jujube honey. Heliyon 2019, 5, e01129. [Google Scholar] [CrossRef] [Green Version]
- Gul, A.; Pehlivan, T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Pizzolante, L.; Toschi, T.G.; Guzzo, F.; Ceoldo, S.; Marconi, A.M.; Andreetta, F.; Levi, M. Phenol content related to antioxidant and antimicrobial activities of Passiflora spp. extracts. Eur. Food Res. Technol. 2006, 223, 102–109. [Google Scholar] [CrossRef]
- Akar, Z.; Küçük, M.; Doğan, H. A new colorimetric DPPH(•) scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J. Enzyme Inhib. Med. Chem. 2017, 32, 640–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, A.; Rigelhof, F.; Miller, E. Medallion Laboratories Analytical Progress: Antioxidant Activity; DeVries, J., Ed.; Medallion Laboratories: Minneapolis, MN, USA, 2001; Volume 19, pp. 1–6. [Google Scholar]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Hostalkova, A.; Klingelhofer, I.; Morlock, G.E. Comparison of an HPTLC method with the Reflectoquant assay for rapid determination of 5-hydroxymethylfurfural in honey. Anal. Bioanal. Chem. 2013, 405, 9207–9218. [Google Scholar] [CrossRef]
- Stanek, N.; Jasicka-Misiak, I. HPTLC Phenolic Profiles as Useful Tools for the Authentication of Honey. (Report). Food Anal. Methods 2018, 11, 2979. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Umeda, N.; Maeda, A.; Matsumoto, D.; Kitamoto, N.; Kikuzaki, H. Identification of a novel glycoside, leptosin, as a chemical marker of manuka honey. J. Agric. Food Chem. 2012, 60, 3418–3423. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Development and validation of an HPTLC–DPPH assay and its application to the analysis of honey. JPC J. Planar Chromat. 2020, 33, 301–311. [Google Scholar] [CrossRef]
- Escriche, I.; Visquert, M.; Juan-Borrás, M.; Fito, P. Influence of simulated industrial thermal treatments on the volatile fractions of different varieties of honey. Food Chem. 2009, 112, 329–338. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014, 142, 135–143. [Google Scholar] [CrossRef]
- Bucekova, M.; Juricova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Canini, A.; Majtan, J. Effect of thermal liquefying of crystallised honeys on their antibacterial activities. Food Chem. 2018, 269, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S. Changes of antioxidant activity and formation of 5-hydroxymethylfurfural in honey during thermal and microwave processing. Food Chem. 2013, 141, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, C.; Li, C.; Huang, Z.Y.; Miao, X. Pathway of 5-hydroxymethyl-2-furaldehyde formation in honey. J. Food Sci. Technol. 2019, 56, 2417–2425. [Google Scholar] [CrossRef]
- Locher, C.; Neumann, J.; Sostaric, T. Authentication of honeys of different floral origins via high-performance thin-layer chromatographic fingerprinting. JPC J. Planar Chromat. 2017, 30, 57–62. [Google Scholar] [CrossRef]
- Ibrahim, R.S.; Khairy, A.; Zaatout, H.H.; Hammoda, H.M.; Metwally, A.M. Digitally-optimized HPTLC coupled with image analysis for pursuing polyphenolic and antioxidant profile during alfalfa sprouting. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1099, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Locher, C.; Tang, E.; Neumann, J.; Sostaric, T. High-performance thin-layer chromatography profiling of Jarrah and Manuka honeys. JPC J. Planar Chromat. 2018, 31, 181–189. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Almasaudi, S.B.; Abbas, A.T.; Al-Hindi, R.R.; El-Shitany, N.A.; Abdel-Dayem, U.A.; Ali, S.S.; Saleh, R.M.; Al Jaouni, S.K.; Kamal, M.A.; Harakeh, S.M. Manuka Honey Exerts Antioxidant and Anti-Inflammatory Activities That Promote Healing of Acetic Acid-Induced Gastric Ulcer in Rats. Evid. Based Complement. Alternat. Med. 2017, 2017, 5413917. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Datta, N.; Tomás-Barberán, F.A.; Ferreres, F.; Martos, I.; Singanusong, R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Alzahrani, H.A.; Alsabehi, R.; Boukraâ, L.; Abdellah, F.; Bellik, Y.; Bakhotmah, B.A. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules 2012, 17, 10540–10549. [Google Scholar] [CrossRef] [Green Version]
- Karabagias, V.K.; Karabagias, I.K.; Gatzias, I. The impact of different heating temperatures on physicochemical, color attributes, and antioxidant activity parameters of Greek honeys. J. Food Process Eng. 2018, 41, e12668. [Google Scholar] [CrossRef]
- Saxena, S.; Gautam, S.; Sharma, A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010, 118, 391–397. [Google Scholar] [CrossRef]
- Baltrušaitytė, V.; Venskutonis, P.R.; Čeksterytė, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Zarei, M.; Fazlara, A.; Tulabifard, N. Effect of thermal treatment on physicochemical and antioxidant properties of honey. Heliyon 2019, 5, e01894. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Labelled Floral Source | Nectar Source | Supplier and Packaging Date | Reference ID |
|---|---|---|---|
| Manuka | Leptospermum spp. | Barnes Naturals (January 2017) | LEP |
| Coastal Peppermint | Agonis flexuosa | Margaret River Honey Co. (December 2017) | AGO |
| Marri | Corymbia calophylla | ICON Honey (February 2018) | COR |
| Karri | Eucalyptus diversicolor | Zees Bees (2016) | EU1 |
| River Red Gum | Eucalyptus camaldulensis | Capilano (May 2014) | EU2 |
| Multifloral Organic | Unknown | Wescobee (December 2016) | MF1 |
| Multifloral | Unknown | Wescobee (June 2019) | MF2 |
| Unspecified | Unknown | Coles Supermarket (Jan 2019) | UN1 |
| Unspecified | Unknown | Aldi Supermarket (No date provided) | UN2 |
| ID | Bands | Rf | Concentration (ng/5 μL Extracts) | mg Gallic Acid Equivalent (per 100 g Honey) | Total Band Activity (mg Gallic Acid Equivalent per 100 g Honey) |
|---|---|---|---|---|---|
| LEP | 1 | 0.27 | 80.32 | 0.1606 | 0.9685 |
| 2 | 0.47 | 356.08 | 0.7122 | ||
| 3 | 0.83 | 47.85 | 0.0957 | ||
| AGO | 1 * | 0.35 | 352.04 | 0.7041 | 0.8101 |
| 2 * | 0.49 | ||||
| 3 | 0.78 | 53.02 | 0.1060 | ||
| EU1 | 1 | 0.30 | 31.06 | 0.0621 | 0.2244 |
| 2 | 0.46 | 34.46 | 0.0689 | ||
| 3 | 0.80 | 46.67 | 0.0933 | ||
| COR | 1 | 0.43 | 113.67 | 0.2273 | 0.3615 |
| 2 | 0.81 | 67.09 | 0.1342 | ||
| EU2 | 1 | 0.08 | 47.42 | 0.0948 | 0.5041 |
| 2 | 0.41 | 129.55 | 0.2591 | ||
| 3 | 0.80 | 75.06 | 0.1501 | ||
| MF1 | 1 | 0.09 | 32.84 | 0.0657 | 0.3638 |
| 2 | 0.31 | 34.08 | 0.0682 | ||
| 3 | 0.48 | 54.53 | 0.1091 | ||
| 4 | 0.81 | 60.47 | 0.1209 | ||
| MF2 | 1 | 0.46 | 64.50 | 0.1290 | 0.2527 |
| 2 | 0.81 | 61.83 | 0.1237 | ||
| UN1 | 1 | 0.08 | 22.02 | 0.0440 | 0.1831 |
| 2 | 0.51 | 51.13 | 0.1022 | ||
| 3 | 0.81 | 18.50 | 0.0369 | ||
| UN2 | Not detected | ||||
| ID | Antioxidant Bands (Rf) | Floral Fingerprint Bands (Rf) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| After Development | After Derivatisation | ||||||||
| R 254 | R 366 | T White | R 366 | ||||||
| Rf | Colour | Rf | Colour | Rf | Colour | Rf | Colour | ||
| LEP | 0.27 | 0.24 | BRB | 0.12 | LY | 0.25 | 0.20 | BRB | |
| 0.47 | 0.35 | 0.25 | BRB | 0.34 | G | 0.25 | BRB | ||
| 0.83 | 0.52 | 0.33 | LB | 0.41 | O | 0.33 | |||
| 0.47 | Y | 0.42 | |||||||
| AGO | 0.35 | 0.23 | 0.11 | Y | 0.10 | 0.11 | |||
| 0.49 | 0.33 | BRB | 0.19 | LY | 0.25 | 0.23 | |||
| 0.78 | 0.39 | 0.32 | BRB | 0.39 | DB | 0.32 | G | ||
| 0.52 | 0.42 | LB | 0.47 | BW | 0.38 | BW | |||
| 0.48 | R | ||||||||
| EU1 | 0.30 | 0.33 | 0.33 | LB | 0.32 | 0.32 | |||
| 0.46 | 0.41 | 0.40 | |||||||
| 0.80 | 0.46 | 0.52 | |||||||
| COR | 0.43 | 0.37 | 0.25 | 0.37 | P | 0.25 | |||
| 0.81 | 0.41 | 0.32 | LB | 0.41 | R | 0.37 | LBW | ||
| 0.47 | 0.47 | O | 0.41 | DBW | |||||
| 0.45 | DB | ||||||||
| EU2 | 0.08 | 0.34 | 0.24 | 0.10 | B | ||||
| 0.41 | 0.41 | 0.33 | G | 0.36 | DBW | ||||
| 0.80 | 0.46 | 0.40 | R | 0.40 | DBW | ||||
| 0.47 | BW | 0.46 | DBW | ||||||
| MF1 | 0.09 | 0.34 | 0.33 | LB | 0.33 | ||||
| 0.31 | 0.42 | 0.36 | |||||||
| 0.48 | 0.46 | 0.41 | |||||||
| 0.81 | |||||||||
| MF2 | 0.46 | 0.33 | 0.11 | 0.20 | 0.23 | B | |||
| 0.81 | 0.41 | 0.24 | BRB | 0.36 | O | 0.37 | BW | ||
| 0.46 | 0.32 | LB | 0.41 | LB | 0.41 | DBW | |||
| 0.46 | B | 0.47 | DR | ||||||
| 0.52 | |||||||||
| UN1 | 0.08 | 0.24 | 0.25 | BW | 0.37 | ||||
| 0.51 | 0.34 | 0.23 | 0.33 | G | 0.41 | BW | |||
| 0.81 | 0.40 | 0.32 | 0.40 | BW | 0.46 | BW | |||
| 0.54 | |||||||||
| UN2 | Not Detected | 0.33 | 0.31 | Very faint; not easily distinguishable | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant HPTLC-DPPH Fingerprinting of Honeys and Tracking of Antioxidant Constituents upon Thermal Exposure. Foods 2021, 10, 357. https://doi.org/10.3390/foods10020357
Islam MK, Sostaric T, Lim LY, Hammer K, Locher C. Antioxidant HPTLC-DPPH Fingerprinting of Honeys and Tracking of Antioxidant Constituents upon Thermal Exposure. Foods. 2021; 10(2):357. https://doi.org/10.3390/foods10020357
Chicago/Turabian StyleIslam, Md Khairul, Tomislav Sostaric, Lee Yong Lim, Katherine Hammer, and Cornelia Locher. 2021. "Antioxidant HPTLC-DPPH Fingerprinting of Honeys and Tracking of Antioxidant Constituents upon Thermal Exposure" Foods 10, no. 2: 357. https://doi.org/10.3390/foods10020357