NMR Characterization of Ten Apple Cultivars from the Piedmont Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemicals
2.3. Sample Preparation
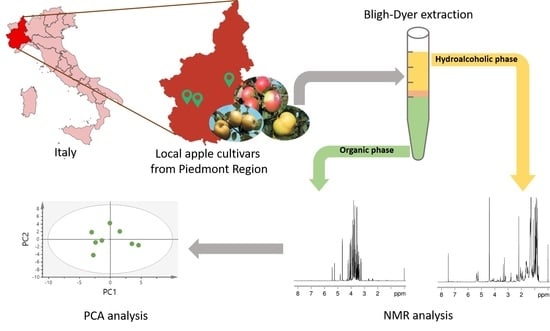
2.4. Extraction Procedure for NMR Analysis
2.5. Metabolic Profile by NMR Analysis
2.6. Multivariate Statistical Analysis
3. Results
3.1. Assignments of Aqueous and Organic Extracts
3.2. Metabolite Profiles of Apple Cultivars
3.2.1. Sugars and Polyols
3.2.2. Organic Acids
3.2.3. Amino Acids
3.2.4. Miscellaneous
3.2.5. β-Sitosterol
3.2.6. Fatty Acids
3.2.7. Polar Lipids
3.3. Multivariate Statistycal Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO FAOSTAT Data. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 18 May 2020).
- U.S. Department of Agriculture Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 18 May 2020).
- Lee, K.W.; Kim, Y.J.; Kim, D.O.; Lee, H.J.; Lee, C.Y. Major Phenolics in Apple and Their Contribution to the Total Antioxidant Capacity. J. Agric. Food Chem. 2003, 51, 6516–6520. [Google Scholar] [CrossRef] [PubMed]
- Francini, A.; Sebastiani, L. Phenolic compounds in apple (Malus x domestica borkh.): Compounds characterization and stability during postharvest and after processing. Antioxidants 2013, 2, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Wikiera, A.; Mika, M.; Grabacka, M. Multicatalytic enzyme preparations as effective alternative to acid in pectin extraction. Food Hydrocoll. 2015, 44, 156–161. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Valorisation of fruit by-products: Production characterization of pectins from fruit peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Caruso, T.; Ciarmiello, L.F.; Cutino, I.; Malvolti, M.E.; Murri, G.; Piccirillo, P. Atlante dei Fruttiferi Autoctoni Italiani; Crea—Centro di Frutticoltura: Roma, Italy, 2016; Volume III, pp. 1276–1285. [Google Scholar]
- Arnaldi, S.M. Antiche Varietà Piemontesi, le Mele. Available online: https://www.antichevarietapiemontesi.it/mele/ (accessed on 6 August 2020).
- Bounous, G. Antiche Cultivar di Melo in Piemonte. Piemonte, R., Ed.; 2006. Available online: http://hdl.handle.net/2318/15310 (accessed on 18 May 2020).
- Bounous, G.; Melo, D.G.I. Collana Coltura & Cultura; Script, A.R.T., Servizi, E.S.P.A., Eds.; Bayer Crop Science: Bologna, Italy, 2008; pp. 82–111. [Google Scholar]
- Cerutti, A.K.; Bruun, S.; Donno, D.; Beccaro, G.L.; Bounous, G. Environmental sustainability of traditional foods: The case of ancient apple cultivars in Northern Italy assessed by multifunctional LCA. J. Clean. Prod. 2013, 52, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Torello Marinoni, D.; Cerutti, A.K.; Canterino, S.; Bounous, G. Application of sensory, nutraceutical and genetic techniques to create a quality profile of ancient apple cultivars. J. Food Qual. 2012, 35, 169–181. [Google Scholar] [CrossRef]
- Ramirez-Ambrosi, M.; Abad-Garcia, B.; Viloria-Bernal, M.; Garmon-Lobato, S.; Berrueta, L.A.; Gallo, B. A new ultrahigh performance liquid chromatography with diode array detection coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry analytical strategy for fast analysis and improved characterization of phenolic compounds in ap. J. Chromatogr. A 2013, 1316, 78–91. [Google Scholar] [CrossRef]
- Mari, A.; Tedesco, I.; Nappo, A.; Russo, G.L.; Malorni, A.; Carbone, V. Phenolic compound characterisation and antiproliferative activity of “Annurca” apple, a southern Italian cultivar. Food Chem. 2010, 123, 157–164. [Google Scholar] [CrossRef]
- Guo, J.; Yue, T.; Yuan, Y.; Wang, Y. Chemometric Classification of Apple Juices According to Variety and Geographical Origin Based on Polyphenolic Profiles. J. Agric. Food Chem. 2013, 61, 6949–6963. [Google Scholar] [CrossRef]
- Bai, L.; Guo, S.; Liu, Q.; Cui, X.; Zhang, X.; Zhang, L.; Yang, X.; Hou, M.; Ho, C.T.; Bai, N. Characterization of nine polyphenols in fruits of Malus pumila Mill by high-performance liquid chromatography. J. Food Drug Anal. 2016, 24, 293–298. [Google Scholar] [CrossRef]
- Ferruzza, S.; Natella, F.; Ranaldi, G.; Murgia, C.; Rossi, C.; Trošt, K.; Mattivi, F.; Nardini, M.; Maldini, M.; Giusti, A.M.; et al. Nutraceutical improvement increases the protective activity of broccoli sprout juice in a human intestinal cell model of gut inflammation. Pharmaceuticals 2016, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Iaccarino, N.; Varming, C.; Petersen, M.A.; Viereck, N.; Schütz, B.; Toldam-Andersen, T.B.; Randazzo, A.; Engelsen, S.B. Ancient danish apple cultivars—A comprehensive metabolite and sensory profiling of apple juices. Metabolites 2019, 9, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aprea, E.; Gika, H.; Carlin, S.; Theodoridis, G.; Vrhovsek, U.; Mattivi, F. Metabolite profiling on apple volatile content based on solid phase microextraction and gas-chromatography time of flight mass spectrometry. J. Chromatogr. A 2011, 1218, 4517–4524. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.P.; Thomas, F.; Donarski, J.; Ingallina, C.; Circi, S.; Cesare Marincola, F.; Capitani, D.; Mannina, L. Use of NMR applications to tackle future food fraud issues. Trends Food Sci. Technol. 2019, 91, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Belton, P.S.; Delgadillo, I.; Gil, A.M.; Roma, P.; Casuscelli, F.; Colquhoun, I.J.; Dennis, M.J.; Spraul, M. High-field proton NMR studies of apple juices. Magn. Reson. Chem. 1997, 35, 52–60. [Google Scholar] [CrossRef]
- Berregi, I.; del Campo, G.; Caracena, R.; Miranda, J.I. Quantitative determination of formic acid in apple juices by 1H NMR spectrometry. Talanta 2007, 72, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, P.; Ehlers, M.; Weinert, C.H.; Tzvetkova, P.; Silber, M.; Rist, M.J.; Luy, B.; Muhle-Goll, C. Untargeted NMR spectroscopic analysis of the metabolic variety of new apple cultivars. Metabolites 2016, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Sciubba, F.; Di Cocco, M.E.; Gianferri, R.; Capuani, G.; De Salvador, F.R.; Fontanari, M.; Gorietti, D.; Delfini, M. Nuclear Magnetic Resonance-Based Metabolic Comparative Analysis of Two Apple Varieties with Different Resistances to Apple Scab Attacks. J. Agric. Food Chem. 2015, 63, 8339–8347. [Google Scholar] [CrossRef]
- Tomita, S.; Nemoto, T.; Matsuo, Y.; Shoji, T.; Tanaka, F.; Nakagawa, H.; Ono, H.; Kikuchi, J.; Ohnishi-Kameyama, M.; Sekiyama, Y. A NMR-based, non-targeted multistep metabolic profiling revealed l-rhamnitol as a metabolite that characterised apples from different geographic origins. Food Chem. 2015, 174, 163–172. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Mannina, L.; Capitani, D.; Sanzò, G.; Ingallina, C.; Botta, B.; Fornarini, S.; Crestoni, M.E.; Chiavarino, B.; Carradori, S.; et al. A multi-methodological approach in the study of Italian PDO “Cornetto di Pontecorvo” red sweet pepper. Food Chem. 2018, 255, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Capitani, D.; Mannina, L.; Carradori, S.; Locatelli, M.; Di Sotto, A.; Di Giacomo, S.; Toniolo, C.; Pasqua, G.; Valletta, A.; et al. Phytochemical and biological characterization of Italian “sedano bianco di Sperlonga” Protected Geographical Indication celery ecotype: A multimethodological approach. Food Chem. 2020, 309, 125649. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Giusti, A.M.; Mannina, L. New hybrid tomato cultivars: An NMR-based chemical characterization. Appl. Sci. 2020, 10, 1887. [Google Scholar] [CrossRef] [Green Version]
- D’Imperio, M.; Dugo, G.; Alfa, M.; Mannina, L.; Segre, A.L. Statistical analysis on Sicilian olive oils. Food Chem. 2007, 102, 956–965. [Google Scholar] [CrossRef]
- Contessa, C.; Botta, R. Comparison of physicochemical traits of red-fleshed, commercial and ancient apple cultivars. Hortic. Sci. 2016, 43, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Cavanna, M.; Marinoni, D.T.; Bounous, G.; Botta, R. Genetic diversity in ancient apple germplasm from northwest Italy. J. Hortic. Sci. Biotechnol. 2008, 83, 549–554. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Viel, S. Liquid state 1H high field NMR in food analysis. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 66, 1–39. [Google Scholar] [CrossRef]
- Arnaldi, S.M. Piante di melo di Antiche Varietà piemontesi. Available online: http://www.scuolamalva.it/wp-content/uploads/2016/06/Descrizione-piante-melo-TRIO.pdf (accessed on 6 August 2020).
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Fraschetti, C.; Filippi, A.; Di Sotto, A.; Di Giacomo, S.; Mazzoccanti, G.; Gasparrini, F.; et al. Cannabis sativa L. Inflorescences from Monoecious Cultivars Grown in Central Italy: An Untargeted Chemical Characterization from Early Flowering to Ripening. Molecules 2020, 25, 1908. [Google Scholar] [CrossRef]
- Zhu, D.; Shen, Y.; Wei, L.; Xu, L.; Cao, X.; Liu, H.; Li, J. Effect of particle size on the stability and flavor of cloudy apple juice. Food Chem. 2020, 328, 126967. [Google Scholar] [CrossRef]
- Vermathen, M.; Marzorati, M.; Baumgartner, D.; Good, C.; Vermathen, P. Investigation of different apple cultivars by high resolution magic angle spinning NMR. A feasibility study. J. Agric. Food Chem. 2011, 59, 12784–12793. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Petriccione, M.; Rega, P.; Scortichini, M.; Napolitano, A. A reappraisal of traditional apple cultivars from Southern Italy as a rich source of phenols with superior antioxidant activity. Food Chem. 2013, 140, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Miyazawa, T. Separation and determination of glycolipids from edible plant sources by high-performance liquid chromatography and evaporative light-scattering detection. Lipids 1999, 34, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Spano, M.; Sobolev, A.P.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L. Characterization of Local Products for Their Industrial Use : The Case of Italian Potato Cultivars Analyzed by Untargeted and Targeted Methodologies. Foods 2020, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Ticha, A.; Salejda, A.M.; Hyšpler, R.; Matejicek, A.; Paprstein, F.; Zadak, Z.; Cukrów, W.S. Jabłkach Różnych Odmian I Ich Wpływ Na Cechy Sensoryczne. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2015, 22, 137–150. [Google Scholar] [CrossRef]
- Hermann, K.; Bordewick-Dell, U. Fructose in different apple varieties. Implications for apple consumption in persons affected by fructose intolerance. Ernährungs Umschau 2018, 65, 48–52. [Google Scholar] [CrossRef]
- Hecke, K.; Herbinger, K.; Veberič, R.; Trobec, M.; Toplak, H.; Štampar, F.; Keppel, H.; Grill, D. Sugar-, acid- and phenol contents in apple cultivars from organic and integrated fruit cultivation. Eur. J. Clin. Nutr. 2006, 60, 1136–1140. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Ku, K.H.; Jeong, M.C.; Kwon, S., II; Lee, J. Metabolite profiling and antioxidant activity of 10 new early- to mid-season apple cultivars and 14 traditional cultivars. Antioxidants 2020, 9, 443. [Google Scholar] [CrossRef]
- Agricultural Marketing Resource Center Apples. Available online: https://www.agmrc.org/commodities-products/fruits/apples (accessed on 6 August 2020).
- Produce Report Global Trends in Apple Innovation. Available online: https://www.producereport.com/article/global-trends-apple-innovation (accessed on 6 August 2020).
- Petkovsek, M.M.; Stampar, F.; Veberic, R. Parameters of inner quality of the apple scab resistant and susceptible apple cultivars (Malus domestica Borkh.). Sci. Hortic. 2007, 114, 37–44. [Google Scholar] [CrossRef]
- Colaric, M.; Veberic, R.; Stampar, F.; Hudina, M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J. Sci. Food Agric. 2005, 85, 2611–2616. [Google Scholar] [CrossRef]
- Hulme, A.C. The isolation of L-citramalic acid from the peel of the apple fruit. Biochim. Biophys. Acta 1954, 14, 36–43. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.L.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabetes. Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Escarpa, A.; González, M.C. High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. J. Chromatogr. A 1998, 823, 331–337. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Niederberger, K.E.; Tennant, D.R.; Bellion, P. Dietary intake of phloridzin from natural occurrence in foods. Br. J. Nutr. 2020, 123, 942–950. [Google Scholar] [CrossRef]
- Kumar, S.; Sinha, K.; Sharma, R.; Purohit, R.; Padwad, Y. Phloretin and phloridzin improve insulin sensitivity and enhance glucose uptake by subverting PPARγ/Cdk5 interaction in differentiated adipocytes. Exp. Cell Res. 2019, 383, 111480. [Google Scholar] [CrossRef]
- Posma, J.M.; Garcia-Perez, I.; Heaton, J.C.; Burdisso, P.; Mathers, J.C.; Draper, J.; Lewis, M.; Lindon, J.C.; Frost, G.; Holmes, E.; et al. Integrated Analytical and Statistical Two-Dimensional Spectroscopy Strategy for Metabolite Identification: Application to Dietary Biomarkers. Anal. Chem. 2017, 89, 3300–3309. [Google Scholar] [CrossRef] [Green Version]
- Noro, S.; Kudo, N.; Kitzuwa, T. Differences in Sugars and Organic Acids between Red and Yellow Apple Cultivars at Time of Coloring, and Effect of Citramalic Acid on Development of Anthocyanin. J. Jpn. Soc. Hortic. Sci. 1988, 57, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Rudell, D.R.; Mattheis, J.P.; Curry, E.A. Prestorage ultraviolet-white light irradiation alters apple peel metabolome. J. Agric. Food Chem. 2008, 56, 1138–1147. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [Green Version]
- Finamore, A.; Roselli, M.; Donini, L.M.; Brasili, D.E.; Rami, R.; Carnevali, P.; Mistura, L.; Pinto, A.; Giusti, A.M.; Mengheri, E. Supplementation with Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 mixture improves immunity in elderly humans (over 75 years) and aged mice. Nutrition 2019, 63, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Boutaoui, N.; Zaiter, L.; Benayache, F.; Benayache, S.; Cacciagrano, F.; Cesa, S.; Secci, D.; Carradori, S.; Giusti, A.M.; Campestre, C.; et al. Atriplex mollis Desf. aerial parts: Extraction procedures, secondary metabolites and color analysis. Molecules 2018, 23, 1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, Á.; Burlingame, B. Biodiversity and nutrition: A common path toward global food security and sustainable development. J. Food Compos. Anal. 2006, 19, 477–483. [Google Scholar] [CrossRef]







| N° | Cultivar | Maturity Time | Peel | Pulp | Size |
|---|---|---|---|---|---|
| A1 | Canditina | Late October | Smooth, green with red overcolour | Juicy, white/green | Medium |
| A2 | Grigia di Torriana | Mid/late October | Rough, green | Soft, white | Medium |
| A3 | Magnana | Late October/early November | Smooth/rough, white/green with red overcolour | Soft, white/green | Medium |
| A4 | Runsé | Late October/early November | Smooth, red | Juicy, white/pink | Medium |
| A5 | Carla | Mid/late September | Smooth, yellow with red/orange overcolour | Soft, white | Medium/small |
| A6 | Gamba Fina | Early/Mid October | Smooth, yellow/green with red overcolour | Soft, white | Medium/small |
| A7 | Ross Giambon | Mid October | Smooth, yellow/green | Juicy, white | Large |
| A8 | Dominici | Mid October | Rough, yellow/green with red overcolour | Crisp, white | Large |
| A9 | Calvilla | Mid October | Smooth, green with red overcolour | Crisp and juicy, white/green | Medium |
| A10 | Grenoble | Late October | Rough, green | Crisp, white/green | Small |
| Compound | Assignment | 1H (ppm) | Multiplicity (J(Hz)) | 13C (ppm) |
|---|---|---|---|---|
| Carbohydrates | ||||
| α-D-Fructofuranose | C-2 | 105.5 | ||
| CH-3 | 4.13 * | 83.0 | ||
| CH-4 | 4.00 | 77.2 | ||
| CH-5 | 4.07 | 82.4 | ||
| β-D-Fructofuranose | C-2 | 102.6 | ||
| CH-3 | 4.12 * | 76.6 | ||
| CH-4 | 4.12 * | 75.5 | ||
| CH-5 | 3.84 | 81.7 | ||
| CH2-6,6′ | 3.83; 3.68 | |||
| β-D-Fructopyranose | CH2-1,1′ | 3.57; 3.72 | 64.9 | |
| C-2 | 99.2 | |||
| CH-3 | 3.80 | 68.6 | ||
| CH-4 | 3.90 | 70.7 | ||
| CH-5 | 4.01 | 70.2 | ||
| CH2-6,6′ | 3.72; 4.03 | 64.5 | ||
| α-Xylose | CH-1 | 5.20 * | d a (3.8) | 93.4 |
| CH-2 | 3.55 | |||
| CH-3 | 3.65 | |||
| β- Xylose | CH-1 | 4.59 * | d (8.0) | 97.7 |
| CH-2 | 3.54 | |||
| CH-3 | 3.69 | |||
| α-Glucose | CH-1 | 5.24 * | d (3.8) | 93.2 |
| CH-2 | 3.55 | 72.2 | ||
| CH-3 | 3.73 | 73.7 | ||
| CH-4 | 3.42 | 70.3 | ||
| CH-5 | 3.84 | 72.8 | ||
| β-Glucose | CH-1 | 4.66 * | d (8.0) | 97.0 |
| CH-2 | 3.25 | dd b (9.4; 7.9) | 75.1 | |
| CH-3 | 3.51 | 76.9 | ||
| CH-4 | 3.43 | 70.7 | ||
| CH-5 | 3.49 | 76.9 | ||
| CH2-6,6′ | 3.90; 3.75 | 61.8 | ||
| Sucrose | CH-1 (glucose) | 5.42 * | d (3.8) | 93.3 |
| CH-2 | 3.57 | 72.0 | ||
| CH-3 | 3.77 | 73.7 | ||
| CH-4 | 3.47 | 70.1 | ||
| CH-5 | 3.85 | 73.5 | ||
| C-2 (fructose) | 104.8 | |||
| CH-3 | 4.22 | d (8.8) | 77.3 | |
| Rhamnitol | CH3 | 1.28 * | d (6.4) | 20.0 |
| CH-2 | 3.88 | 68.2 | ||
| CH-3 | 3.61 | 74.3 | ||
| Organic acids | ||||
| Acetic acid | α-CH3 | 1.92 * | s c | |
| Citric acid | α,γ-CH | 2.54 * | d (15.2) | 46.7 |
| α’,γ’-CH | 2.67 | 46.7 | ||
| β-C | 76.5 | |||
| 1,5-COOH | 180.8 | |||
| 6-COOH | 183.0 | |||
| Formic acid | HCOOH | 8.46 * | s | |
| Citramalic acid | β-CH3 | 1.33 * | s | 26.5 |
| β-CH2 | 2.44 | d (15.8) | 47.7 | |
| β’-CH2 | 2.74 | d (15.8) | 47.7 | |
| α-C | 75.5 | |||
| 1,4-COOH | 184.2 | |||
| Lactic acid | β-CH3 | 1.33 * | d (7.0) | 21.4 |
| α-CH | 4.12 | 69.8 | ||
| COOH | 183.4 | |||
| Malic acid | α-CH | 4.30 * | dd (9.9; 3.2) | 71.6 |
| β-CH | 2.67 | dd (15.4; 3.2) | 43.9 | |
| β’-CH | 2.37 | dd (15.4; 9.9) | 43.9 | |
| Quinic acid | C-1 | 78.3 | ||
| CH2-2, 2′ | 1.88 *; 2.06 | dd (13.5; 10.8); m | 41.9 | |
| CH-3 | 4.03 | m d | 68.0 | |
| CH-4 | 3.57 | m | 76.2 | |
| CH-5 | 4.16 | m | 71.3 | |
| α-Galacturonic acid | CH-1 | 5.31 | d (3.8) | 93.1 |
| CH-2 | 3.80 | |||
| CH-3 | 3.90 | |||
| CH-4 | 4.29 | |||
| CH-5 | 4.41* | d (1.2) | 72.5 | |
| COOH | 177.1 | |||
| Amino acids | ||||
| Alanine | α-CH | 3.80 | 51.5 | |
| β-CH3 | 1.49 * | d (7.3) | 17.3 | |
| COOH | 176.8 | |||
| Asparagine | α-CH | 4.05 | 52.3 | |
| β,β’-CH2 | 2.88 *; 2.96 | dd (7.4; 16.9); dd (4.3; 12.6) | 35.6 | |
| COOH | 175.5 | |||
| Aspartate | β,β’-CH2 | 2.70; 2.81 * | dd(3.7; 17.4) | |
| γ-Aminobutyrate | α-CH2 | 2.30 * | t e (7.4) | 35.3 |
| β-CH2 | 1.90 | 24.7 | ||
| γ-CH2 | 3.01 | |||
| Isoleucine | γ-CH3 | 1.01 * | d (7.1) | |
| Valine | γ-CH3 | 0.99 * | d (7.1) | |
| γ’-CH3 | 1.05 | d (7.1) | ||
| Miscellaneous metabolites | ||||
| Chlorogenic acid | CH2-2 | 2.20 | ||
| CH-3 | 5.33 | m | 72.2 | |
| CH2-6 | 2.04 | |||
| CH-1′ | 6.42 * | d (16.0) | 115.8 | |
| CH-2′ | 7.67 | d (16.0) | 147.3 | |
| CH-3′ | 7.22 | d (2.0) | 116.3 | |
| CH-6′ | 6.98 | 116.7 | ||
| CH-7′ | 7.15 | 123.8 | ||
| CH2-6, 6′ | 1.97;2.05 | m | 38.5 | |
| Choline | N(CH3)3+ | 3.21 * | s | 55.1 |
| p-Coumaric acid derivative | CH-2,6 | 7.62 * | d (8.8) | 131.9 |
| CH-3,5 | 6.97 | |||
| CH=CH | 7.79; 6.51 | d (16.1) | ||
| Phloretin/Phloridzin | CH-2,6 | 7.16 | d (8.3) | 130.7 |
| CH-3,5 | 6.85 * | d (8.3) | 116.5 | |
| CH-3′,5′ | 6.19; 6.24 | s | 96.6; 97.3 | |
| β-CH2 | 2.92 | 31.0 | ||
| Compound | Assignment | 1H (ppm) | Multiplicity (J(Hz)) | 13C (ppm) |
|---|---|---|---|---|
| Oleic fatty chain | COO | 174.4 | ||
| (C18:1 Δ9) | CH2-2 | 2.30 | 34.6 | |
| CH2-3 | 1.58 | m a | 25.3 | |
| CH2-4,7 | 1.30 | m | 29.5 | |
| CH2-8 | 2.01 | m | 27.6 | |
| CH=CH 9,10 | 5.31 | m | 130.6 | |
| CH2-11 | 2.01 | m | 27.6 | |
| CH2-12,15 | 1.33–1.30 | m | 29.4–30.2 | |
| CH2-16 | 1.28 | m | 31.6 | |
| CH2-17 | 1.26 | m | 23.0 | |
| CH3-18 | 0.84 | t b | 14.4 | |
| Linoleic fatty chain | COO | 174.4 | ||
| (C18:2 Δ9,12) | CH2-2 | 2.30 | 34.6 | |
| CH2-3 | 1.58 | m | 25.3 | |
| CH2-4,7 | 1.32–1.28 | m | 29.5 | |
| CH2-8 | 2.02 | m | 27.6 | |
| CH= 9 | 5.34 | m | 130.6 | |
| CH= 10 | 5.31 | m | 128.6 | |
| CH2-11 | 2.73 * (IDI) | t (6.8) | 26.0 | |
| CH= 12 | 5.31 | m | 128.6 | |
| CH= 13 | 5.34 | m | 130.6 | |
| CH2-14 | 2.02 | m | 27.6 | |
| CH2-15 | 1.29 | m | 29.4 | |
| CH2-16 | 1.29 | m | 31.6 | |
| CH2-17 | 1.23 | m | 23.0 | |
| CH3-18 | 0.85 | t | 14.4 | |
| Linolenic fatty chain | COO | 174.4 | ||
| (C18:3 Δ9,12,15) | CH2-2 | 2.30 | 34.9 | |
| CH2-3 | 1.58 | m | 25.3 | |
| CH2-4,7 | 1.30 | m | 29.5 | |
| CH2-8 | 2.03 | m | 27.6 | |
| CH= 9 | 5.34 | m | 130.6 | |
| CH= 10 | 5.30 | m | 128.6 | |
| CH2 11 | 2.77 * (ITRI) | t (6.2) | 26.0 | |
| CH=CH 12,13 | 5.30 | m | 128.6 | |
| CH2-14 | 2.77 * (ITRI) | t (6.2) | 26.0 | |
| CH= 15 | 5.28 | m | 127.4 | |
| CH= 16 | 5.34 | m | 132.2 | |
| CH2-17 | 2.03 | m | 20.9 | |
| CH3-18 | 0.94 | t (7.6) | 14.4 | |
| Saturated fatty acids | COO | 174.4 | ||
| CH2-2 | 2.28 | 34.6 | ||
| CH2-3 | 1.58 | m | 25.3 | |
| CH2 | 1.28–1.22 | m | 29.6-32.0 | |
| CH2 n-1 | 1.26 | 22.9 | ||
| CH3 n | 0.84 | t | 14.4 | |
| Diacylglycerol moiety | CH-sn 2 | 5.06 | 72.5 | |
| CH-sn 1 | 4.15, 4.33 | 62.5 | ||
| CH-sn 3 | 3.65 | 61.0 | ||
| β-Sitosterol | CH3-18 | 0.66 * (ISTE) | s c | 12.2 |
| Squalene | CH3-a | 1.56 | 16.3 | |
| CH3-b | 1.64 | 25.8 | ||
| CH-c | 5.07 | m | 124.8 | |
| CH2-d | 2.02 | 27.4 | ||
| CH2-e | 1.96 | 40.1 | ||
| 1,2-Diacyl-sn-glycero-3- phosphatidylcholine | N(CH3)3+ | 3.22 * (IPC) | s | 54.5 |
| CH2N+ | 3.75 | 66.4 | ||
| CH2OP | 4.45 | 60.6 | ||
| CH-sn 2 | 5.06 | 72.5 | ||
| CH-sn 1 | 4.15, 4.33 | 62.5 | ||
| CH-sn 3 | 3.65 | 61.0 | ||
| Digalactosyldiacylglycerol | CH’’-1 | 4.87 * (IDGDG) | d d(3.8) | 99.8 |
| CH’’-2 | 3.77 | 69.2 | ||
| CH’’-3 | 3.69 | 70.6 | ||
| CH’’-4 | 3.91 | 70.2 | ||
| CH’-1 | 4.19 | 104.5 | ||
| CH’-2,3 | 3.51–3.53 | |||
| CH’-4 | 3.90 | |||
| CH-sn 2 | 5.06 | 72.5 | ||
| CH-sn 1 | 4.15, 4.33 | 62.5 | ||
| CH-sn 3 | 3.65 | 61.0 |
| Cultivar | Reference | Fru/Glc | Fru/Suc | Sugar/Acid |
|---|---|---|---|---|
| Golden Delicious | [18] | 2.0 | 2.3 | 34 |
| Golden Delicious | [44] | 2.9 | 1.8 | |
| Golden Delicious | [45] | 3.6 | 1.9 | 60 |
| Fuji | [18] | 1.7 | 2.8 | 42 |
| Fuji | [44] | 15.0 | 2.0 | |
| Fuji | [45] | 1.9 | 3.0 | 90 |
| Jonagold | [18] | 1.7 | 2.6 | 31 |
| Jonagold | [44] | 7.3 | 1.1 | |
| Jonagold | [45] | 3.2 | 1.8 | 127 |
| A1 | Present work | 2.3 | 2.4 | 57 |
| A2 | Present work | 1.8 | 3.2 | 61 |
| A3 | Present work | 3.6 | 1.3 | 44 |
| A4 | Present work | 3.5 | 3.1 | 43 |
| A5 | Present work | 2.9 | 6.5 | 69 |
| A6 | Present work | 4.2 | 2.0 | 42 |
| A7 | Present work | 3.6 | 2.1 | 49 |
| A8 | Present work | 3.8 | 2.3 | 37 |
| A9 | Present work | 4.3 | 1.5 | 37 |
| A10 | Present work | 3.2 | 1.3 | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Matteo, G.; Spano, M.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L.; Ingallina, C.; Sobolev, A.P. NMR Characterization of Ten Apple Cultivars from the Piedmont Region. Foods 2021, 10, 289. https://doi.org/10.3390/foods10020289
Di Matteo G, Spano M, Esposito C, Santarcangelo C, Baldi A, Daglia M, Mannina L, Ingallina C, Sobolev AP. NMR Characterization of Ten Apple Cultivars from the Piedmont Region. Foods. 2021; 10(2):289. https://doi.org/10.3390/foods10020289
Chicago/Turabian StyleDi Matteo, Giacomo, Mattia Spano, Cristina Esposito, Cristina Santarcangelo, Alessandra Baldi, Maria Daglia, Luisa Mannina, Cinzia Ingallina, and Anatoly P. Sobolev. 2021. "NMR Characterization of Ten Apple Cultivars from the Piedmont Region" Foods 10, no. 2: 289. https://doi.org/10.3390/foods10020289