Investigation of the Maturity Evaluation Indicator of Honey in Natural Ripening Process: The Case of Rape Honey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Main Reagents and Equipments
2.2.1. Main Reagents
2.2.2. Main Equipments
2.3. Physicochemical Parameters of Honey Samples
2.4. Determination of Total Phenolic Content (TPC), Total Flavonoids Content (TFC) and Proline Content
2.5. Determination of Antioxidant Capacity
2.5.1. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radical Scavenging Ability
2.5.2. Ferric Reducing/Antioxidant Power (FRAP) Assay
2.5.3. Reducing Power
2.6. Analysis of Volatile Constituents by Headspace Gas Chromatography-Ion Mobility Spectrometry (HS-GC-IMS)
2.7. Statistical Data Analysis
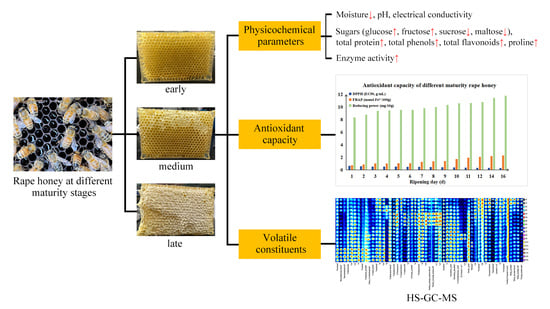
3. Results
3.1. Analysis of Physicochemical Properties
3.2. Antioxidant Analysis and Proline Content
3.3. Analysis of Volatile Profiles
4. Discussion
4.1. Effects of Physicochemical Indexes on Evaluation of Honey Maturity
4.2. Effects of Bioactive Constituents and Antioxidant Capacity on Evaluation of Honey Maturity
4.3. Effects of Volatile Compounds on Evaluation of Honey Maturity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alimentarius, C. Revised codex standard for honey. Codex Stan 2001, 12, 1982. [Google Scholar]
- European Community. Council Directive 2001/110/EC of December 2001 relating to honey. Off. J. Eur. Communities 2002, L10/47–L10/52. [Google Scholar]
- Apimondia Statement on Honey Fraud. Available online: http://www.asianapiculture.org/newsshow.asp?id=1257&big=1 (accessed on 10 October 2021).
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.G.; Marcazzan, G.L.; Piro, R.; et al. Honey quality and international regulatory standards: Review by the International Honey Commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.J.; Le, C.B.; Yang, W.C. The correlation between proline content in honey and its maturity. Apic. China 2015, 66, 52–54. [Google Scholar]
- Gil, M.I.; Ferreres, F.; Ortiz, A.; Subra, E.; Tomas-Barberan, F.A. Plant phenolic metabolites and floral origin of rosemary honey. J. Agric. Food Chem. 1995, 43, 2833–2838. [Google Scholar] [CrossRef]
- Truchado, P.; Tourn, E.; Gallez, L.M.; Moreno, D.A.; Ferreres, F.; Tomás-Barberán, F.A. Identification of botanical biomarkers in Argentinean Diplotaxis honeys: Flavonoids and glucosinolates. J. Agric. Food Chem. 2010, 58, 12678–12685. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.D.; Gu, Z.Y.; Lin, C.F. Studies on protein and enzyme of rape honey. J. Chin. Inst. Food Sci. Technol. 2017, 17, 246–252. [Google Scholar]
- Ma, T.; Zhao, H.; Liu, C.; Zhu, M.; Gao, H.; Cheng, N.; Cao, W. Discrimination of natural mature acacia honey based on multi-physicochemical parameters combined with chemometric analysis. Molecules 2019, 24, 2674. [Google Scholar] [CrossRef] [Green Version]
- Gismondi, A.; De Rossi, S.; Canuti, L.; Novelli, S.; Di Marco, G.; Fattorini, L.; Canini, A. From Robinia pseudoacacia L. nectar to acacia monofloral honey: Biochemical changes and variation of biological properties. J. Sci. Food Agric. 2018, 98, 4312–4322. [Google Scholar] [CrossRef]
- Guo, N.N.; Zhao, L.W.; Zhao, Y.Z.; Li, Q.Q.; Xue, X.F.; Wu, L.M.; Escalada, M.G.; Wang, K.; Peng, W.J. Comparison of the chemical composition and biological activity of mature and immature honey: An HPLC/QTOF/MS-based metabolomic approach. J. Agric. Food Chem. 2020, 68, 4062–4071. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, D.P.; Tian, H.Y.; Su, S.F.; Liu, Y.M.; Liu, Y.R.; Liu, W.; Ming, S.X.; Wang, J. Comparative study about phenolic components between mature and immature acacia honey collected in Shandong area. Food Ferment. Ind. 2018, 44, 256–258. [Google Scholar]
- Sun, J.; Zhao, H.A.; Wu, F.H.; Zhu, M.; Zhang, Y.; Cheng, N.; Xue, X.F.; Wu, L.M.; Cao, W. Molecular mechanism of mature honey formation by GC-MS- and LC-MS-based metabolomics. J. Agric. Food Chem. 2021, 69, 3362–3370. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef] [Green Version]
- Naef, R.; Jaquier, A.; Velluz, A.; Bachofen, B. From the linden flower to linden honey-volatile constituents of linden nectar, the extract of bee-stomach and ripe honey. Chem. Biodivers. 2004, 1, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Zhang, S.B.; Zhang, H.M.; Zhao, G.Y.; Li, X.M.; Wei, Y.H.; Cui, Z.Y. Determination of volatile components in buckwheat honey and correlation analysis with honey maturity. Food Sci. 2020, 41, 222–230. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Si, J.J.; Li, S.S.; Zhang, G.Z.; Wang, S.; Zheng, H.Q.; Hu, F.L. Chemical analyses and antimicrobial activity of nine kinds of unifloral Chinese honeys compared to Manuka honey (12+ and 20+). Molecules 2021, 26, 2778. [Google Scholar] [CrossRef]
- De Almeida-Muradian, L.B.; Barth, O.M.; Dietemann, V.; Eyer, M.; de Freitasd, A.D.S.; Martel, A.C.; Marcazzan, G.L.; Marchese, C.M.; Mucignat-Caretta, C.; Pascual-Maté, A.; et al. Standard methods for Apis mellifera honey research. J. Apic. Res. 2020, 59, 1–62. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 1990. [Google Scholar]
- Bentabol Manzanares, A.; Hernández García, Z.; Rodríguez Galdón, B.; Rodríguez Rodríguez, E.; Díaz Romero, C. Physicochemical characteristics of minor monofloral honeys from Tenerife, Spain. LWT-Food Sci. Technol. 2014, 55, 572–578. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Chen, Y.F.; Wu, Y.Q.; Si, J.J.; Zhang, C.P.; Zheng, H.Q.; Hu, F.L. Discrimination of the entomological origin of honey according to the secretions of the bee (Apis cerana or Apis mellifera). Food Res. Int. 2019, 116, 362–369. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Low, N.H.; Vong, K.V.; Sporns, P. A new enzyme, beta-glucosidase, in honey. J. Apic. Res. 1986, 25, 178–181. [Google Scholar] [CrossRef]
- Yuan, Y.; Deng, Z.Y.; Zhang, B.; Li, G.Y.; Zhang, J.W.; Liu, R.; Li, H.Y. Quality evaluation and geographical classification of immature rape and acacia honeys in China. J. Sci. Food Agric. 2021, 101, 5446–5456. [Google Scholar] [CrossRef] [PubMed]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Tang, Z.Z.; Wang, Y.S.; Yang, J.Y.; Xiao, Y.R.; Cai, Y.; Wan, Y.J.; Chen, H.; Yao, H.P.; Shan, Z.; Li, C.L.; et al. Isolation and identification of flavonoid-producing endophytic fungi from medicinal plant Conyza blinii H. Lév that exhibit higher antioxidant and antibacterial activities. PeerJ 2020, 8, e8978. [Google Scholar] [CrossRef] [Green Version]
- Turkmen, N.; Sari, F.; Poyrazoglu, E.S.; Velioglu, Y.S. Effects of prolonged heating on antioxidant activity and colour of honey. Food Chem. 2006, 95, 653–657. [Google Scholar] [CrossRef]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Atmani, D.; Chaher, N.; Berboucha, M.; Ayouni, K.; Lounis, H.; Boudaoud, H.; Debbache, N.; Atmani, D. Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chem. 2009, 112, 303–309. [Google Scholar] [CrossRef]
- Wang, X.R.; Rogers, K.M.; Li, Y.; Yang, S.P.; Chen, L.Z.; Zhou, J.H. Untargeted and targeted discrimination of honey collected by Apis cerana and Apis mellifera based on volatiles using HS-GC-IMS and HS-SPME-GC-MS. J. Agric. Food Chem. 2019, 67, 12144–12152. [Google Scholar] [CrossRef]
- Feng, D.; Wang, J.; Ji, X.J.; Min, W.X.; Yan, W.J. HS-GC-IMS detection of volatile organic compounds in yak milk powder processed by different drying methods. LWT-Food Sci. Technol. 2021, 141, 110855. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Vyviurska, O.; Chlebo, R.; Pysarevska, S.; Spanik, I. The tracing of VOC composition of acacia honey during ripening stages by comprehensive two-dimensional gas chromatography. Chem. Biodivers. 2016, 13, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.E.A. Factors affecting the physicochemical properties and chemical composition of bee’s honey. Food Rev. Int. 2020, 1–12. [Google Scholar] [CrossRef]
- Iqbal, S.; Sukhmeet, S. Honey moisture reduction and its quality. J. Food Sci. Technol. 2018, 55, 3861–3871. [Google Scholar] [CrossRef]
- Wen, Y.Q.; Wang, L.; Jin, Y.; Zhang, J.Z.; Su, L.; Zhang, X.L.; Zhou, J.H.; Li, Y. The microbial community dynamics during the vitex honey ripening process in the honeycomb. Front. Microb. 2017, 8, 1649. [Google Scholar] [CrossRef]
- Bobis, O.; Moise, A.R.; Ballesteros, I.; Reyes, E.S.; Duran, S.S.; Sanchez-Sanchez, J.; Cruz-Quintana, S.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Eucalyptus honey: Quality parameters, chemical composition and health-promoting properties. Food Chem. 2020, 325, 126870. [Google Scholar] [CrossRef]
- Do Nascimento, K.S.; Sattler, J.A.G.; Macedo, L.F.L.; Gonzalez, C.V.S.; de Melo, I.L.P.; Araujo, E.D.; Granato, D.; Sattler, A.; De Almeida-Muradian, L.B. Phenolic compounds, antioxidant capacity and physicochemical properties of Brazilian Apis mellifera honeys. LWT-Food Sci. Technol. 2018, 91, 85–94. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, L.; Fernández-González, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsi, F.; Ferrari, G.; Hamdi, S. Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab. J. Chem. 2018, 11, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, L.D.C.; Azeredo, M.A.A.; de Souza, S.R.; Dutra, V.M.L. Protein contents and physicochemical properties in honey samples of Apis mellifera of different floral origins. Food Chem. 2003, 80, 249–254. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; de Almeida-Muradian, L.B.; Sancho, M.T.; Pascual-Mate, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2018, 57, 5–37. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Calla, B. Honey as a functional food for Apis mellifera. Annu. Rev. Entomol. 2021, 66, 185–208. [Google Scholar] [CrossRef]
- Yi, S.Q.; Zheng, H.Q.; Zhang, C.P.; Wei, W.T.; Hu, F.L. Determination of beta-glucosidase activity and source in honey. Food Sci. 2010, 31, 241–244. Available online: https://caod.oriprobe.com/articles/38055374/feng_mi_zhong____pu_tao_tang_zuo_mei_huo_xing_ce_d.htm (accessed on 10 October 2021).
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Gheldof, N.; Engeseth, N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef]
- Szczęsna, T.; Rybak-Chmielewska, H.; Waś, E.; Kachaniuk, K.; Teper, D. Characteristics of Polish unifloral honeys. I. rape honey (Brassica napus L. var. oleifera Metzger). J. Apic. Sci. 2011, 55, 111–119. [Google Scholar]
- Saxena, S.; Gautam, S.; Sharma, A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010, 118, 391–397. [Google Scholar] [CrossRef]
- Da Costa, I.F.; Toro, M.J.U. Evaluation of the antioxidant capacity of bioactive compounds and determination of proline in honeys from Pará. J. Food Sci. Technol. 2021, 58, 1900–1908. [Google Scholar] [CrossRef]
- Wang, S.Q.; Chen, H.T.; Sun, B.G. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.Y.; Qin, Y.Y.; Jiang, L.W.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Kallithraka, S.; Arvanitoyannis, I.S.; Kefalas, P.; El-Zajouli, A.; Soufleros, E.; Psarra, E. Instrumental and sensory analysis of Greek wines; implementation of principal component analysis (PCA) for classification according to geographical origin. Food Chem. 2015, 73, 501–514. [Google Scholar] [CrossRef]
- Wang, X.R.; Yang, S.P.; He, J.N.; Chen, L.Z.; Zhang, J.Z.; Jin, Y.; Zhou, J.H.; Zhang, Y.X. A green triple-locked strategy based on volatile-compound imaging, chemometrics, and markers to discriminate winter honey and sapium honey using headspace gas chromatography-ion mobility spectrometry. Food Res. Int. 2019, 119, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Radovic, B.S.; Careri, M.; Mangia, A.; Musci, M.; Gerboles, M.; Anklam, E. Contribution of dynamic headspace GC-MS analysis of aroma compounds to authenticity testing of honey. Food Chem. 2001, 72, 511–520. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Zeng, Z.J.; Zheng, Y.L.; Wu, X.B. Composition analysis of honey from cinnamomum obtusifolium (roxb.) flowers grown in Jiangxi. Food Sci. 2012, 33, 195–199. [Google Scholar]
- Chen, T.T.; Hu, Q.; Tang, J.; Wang, X.M.; Liu, B.; Chen, L. Analysis of volatile components of four kinds of honey in western Sichuan plateau based on electronic nose and gas chromatography-mass spectrometry. Food Sci. 2018, 39, 233–239. [Google Scholar]



| Ripening Day (d) | Capping Ratio (%) | Predominant Pollen (%) | Moisture (%) | Glucose (%) | Fructose (%) | Sucrose (%) | Maltose (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0 g | 85.62 ± 3.6 ab | 26.9 ± 0.6 a | 32 ± 2 a | 28 ± 1 a | 0.85 ± 0.03 a | 1.63 ± 0.03 a |
| 2 | 0 g | 83.15 ± 2.1 ab | 24.9 ± 0.2 a | 34 ± 4 a | 29 ± 1 a | 0.78 ± 0.04 ab | 1.54 ± 0.03 b |
| 3 | 0 g | 88.36 ± 1.9 ab | 22 ± 1 b | 35 ± 4 a | 30 ± 2 a | 0.83 ± 0.05 a | 1.32 ± 0.02 c |
| 4 | 0 g | 86.15 ± 4.5 ab | 22 ± 1 bc | 36 ± 2 a | 30 ± 2 a | 0.72 ± 0.01 b | 1.24 ± 0.01 d |
| 5 | 2.3 ± 0.2 fg | 83.24 ± 3.2 ab | 21 ± 1 bcd | 35 ± 6 a | 30 ± 3 a | 0.60 ± 0.03 c | 1.16 ± 0.01 e |
| 6 | 9.6 ± 0.7 f | 85.37 ± 3.3 ab | 20.1 ± 0.6 bcd | 35 ± 6 a | 29 ± 2 a | 0.61 ± 0.04 c | 1.08 ± 0.02 f |
| 7 | 22 ± 2 e | 89.43 ± 1.6 ab | 19.9 ± 0.9 bcd | 35 ± 4 a | 30 ± 3 a | 0.57 ± 0.05 c | 1.11 ± 0.01 ef |
| 8 | 27 ± 4 e | 80.56 ± 2.8 b | 20.1 ± 0.5 bcd | 34 ± 5 a | 30 ± 3 a | 0.47 ± 0.01 d | 1.02 ± 0.01 g |
| 9 | 47 ± 3 d | 90.48 ± 3.1 a | 19.5 ± 0.8 cd | 35 ± 3 a | 31 ± 1 a | 0.46 ± 0.03 d | 0.84 ± 0.05 h |
| 10 | 69 ± 6 c | 81.34 ± 4.1 b | 20 ± 1 cd | 35.4 ± 0.8 a | 31 ± 2 a | 0.46 ± 0.01 d | 0.87 ± 0.01 h |
| 11 | 78 ± 6 b | 85.26 ± 2.2 ab | 20 ± 1 cd | 36 ±3 a | 31 ± 1 a | 0.45 ± 0.00 d | 0.88 ± 0.01 h |
| 12 | 85 ± 5 b | 88.36 ± 4.3 ab | 19.2 ± 0.2 d | 35 ± 5 a | 33 ± 2 a | 0.45 ± 0.02 d | 0.73 ± 0.02 i |
| 14 | 100.0 ± 0.0 a | 83.29 ± 2.7 ab | 18.5 ± 0.2 d | 37 ± 4 a | 35 ± 4 a | 0.43 ± 0.00 d | 0.67 ± 0.02 j |
| 16 | 100.0 ± 0.0 a | 82.47 ± 1.8 ab | 18.5 ± 0.7 d | 39 ± 1 a | 35 ± 2 a | 0.41 ± 0.03 d | 0.67 ± 0.02 j |
| Ripening Day (d) | Electrical Conductivity (μs/cm) | pH | Total Protein (mg/g) | Diastase (DN) | Invertase (mg/g·h) | Glucose Oxidase (mg/g·0.5 h) | β-glucosidase (U/g) |
| 1 | 179 ± 5 a | 3.72 ± 0.01 a | 6.7 ± 0.5 c | 16 ± 4 c | 44 ± 6 e | 133 ± 41 e | 0.85 ± 0.02 c |
| 2 | 171 ± 2 a | 3.60 ± 0.01 cde | 6.9 ± 0.6 bc | 19 ± 4 bc | 47 ± 5 e | 165 ± 15 de | 0.89 ± 0.06 c |
| 3 | 162 ± 18 a | 3.66 ± 0.02 abc | 7.5 ± 0.6 abc | 21 ± 6 abc | 85 ± 9 de | 189 ± 42 cde | 0.88 ± 0.03 c |
| 4 | 164 ± 2 a | 3.69 ± 0.03 ab | 7.4 ± 0.6 abc | 20 ± 4 abc | 112 ± 15 cd | 192 ± 29 cde | 0.91 ± 0.02 c |
| 5 | 152 ± 15 a | 3.63 ± 0.00 bcd | 7.4 ± 0.6 abc | 21 ± 4 abc | 117 ± 27 cd | 240 ± 7 bcd | 0.92 ± 0.04 c |
| 6 | 155 ± 10 a | 3.55 ± 0.04 e | 7.9 ± 0.6 abc | 22 ± 3 abc | 123 ± 26 bcd | 252 ± 5 abcd | 0.94 ± 0.04 c |
| 7 | 159 ± 11 a | 3.58 ± 0.03 de | 8.5 ± 0.7 abc | 23 ± 2 abc | 131.3 ± 0.4 abcd | 280.0 ± 0.1 abc | 0.95 ± 0.03 c |
| 8 | 162 ± 14 a | 3.61 ± 0.03 cde | 8.4 ± 0.7 abc | 23 ± 2 abc | 130 ± 20 abcd | 260 ± 52 abcd | 1.11 ± 0.02 b |
| 9 | 162 ± 9 a | 3.56 ± 0.01 de | 8.1 ± 0.7 abc | 24 ± 3 abc | 149 ± 20 abc | 284 ± 15 abc | 1.17 ± 0.02 b |
| 10 | 160 ± 11 a | 3.54 ± 0.03 e | 8.4 ± 0.7 abc | 24.9 ± 0.3 abc | 162 ± 21 abc | 307 ± 7 ab | 1.12 ± 0.04 b |
| 11 | 166 ± 6 a | 3.58 ± 0.00 de | 8.7 ± 0.7 ab | 25.2 ± 0.4 ab | 167 ± 27 abc | 353 ± 66 a | 1.21 ± 0.03 b |
| 12 | 164 ± 7 a | 3.56 ± 0.05 e | 8.6 ± 0.7 abc | 26.06 ± 0.01 ab | 163 ± 31 abc | 352 ± 42 a | 1.21 ± 0.02 b |
| 14 | 156 ± 1 a | 3.59 ± 0.01 cde | 8.6 ± 0.7 abc | 29 ± 1 a | 180 ± 24 ab | 305 ± 53 ab | 1.40 ± 0.07 a |
| 16 | 161 ± 8 a | 3.60 ± 0.02 cde | 8.8 ± 0.7 a | 29 ± 1 a | 189 ± 19 a | 276 ± 32 abc | 1.41 ± 0.01 a |
| Ripening Day (d) | TPC (mg GAE/100 g) | TFC (mg QE/100 g) | Proline (mg/kg) | DPPH (EC50, g/mL) | FRAP (mmol Fe2+/100 g) | Reducing Power (mg/100 g) |
|---|---|---|---|---|---|---|
| 1 | 23.8 ± 0.9 b | 24 ± 2 c | 212 ± 13 c | 0.63 ± 0.07 a | 0.74 ± 0.08 f | 83 ± 4 e |
| 2 | 24 ± 2 b | 26 ± 2 bc | 245 ± 12 bc | 0.58 ± 0.01 ab | 0.86 ± 0.09 f | 88 ± 6 de |
| 3 | 24.5 ± 0.4 b | 28 ± 3 abc | 261 ± 32 abc | 0.53 ± 0.03 bc | 1.03 ± 0.04 ef | 94 ± 6 cde |
| 4 | 25.1 ± 0.6 b | 29 ± 3 abc | 262 ± 17 abc | 0.51 ± 0.02 bcd | 1.03 ± 0.04 ef | 94 ± 5 cde |
| 5 | 26 ± 2 ab | 31 ± 3 abc | 265 ± 12 abc | 0.51 ± 0.02 bcd | 1.06 ± 0.08 def | 95 ± 7 cde |
| 6 | 27 ± 2 ab | 31 ± 4 abc | 289 ± 35 ab | 0.48 ± 0.00 cde | 1.06 ± 0.06 def | 95 ± 7 cde |
| 7 | 28 ± 2 ab | 32 ± 1 abc | 299 ± 8 ab | 0.48 ± 0.01 cdef | 1.3 ± 0.2 de | 98 ± 7 bcde |
| 8 | 27 ± 1 ab | 32 ± 2 abc | 292 ± 18 ab | 0.44 ± 0.04 def | 1.4 ± 0.1 cde | 100 ± 11 abcde |
| 9 | 27.4 ± 0.9 ab | 33 ± 2 ab | 294 ± 13 ab | 0.43 ± 0.00 efg | 1.40 ± 0.02 cd | 103 ± 8 abcd |
| 10 | 27.3 ± 0.5 ab | 33 ± 3 ab | 298 ± 10 ab | 0.40 ± 0.01 fgh | 1.7 ± 0.1 bc | 106 ± 2 abcd |
| 11 | 30 ± 2 ab | 34 ± 4 ab | 306 ± 22 ab | 0.36 ± 0.02 ghi | 1.9 ± 0.2 ab | 106 ± 2 abc |
| 12 | 30 ± 4 ab | 34 ± 3 ab | 306 ± 30 ab | 0.34 ± 0.03 hi | 2.1 ± 0.2 ab | 108 ± 3 abc |
| 14 | 32 ± 5 a | 33 ± 4 ab | 314 ± 46 a | 0.33 ± 0.01 hi | 2.2 ± 0.2 a | 114 ± 5 ab |
| 16 | 32 ± 3 a | 35 ± 4 a | 318 ± 18 a | 0.31 ± 0.03 i | 2.3 ± 0.1 a | 118 ± 7 a |
| TPC | TFC | Proline | DPPH | FRAP | Reducing Power | |
|---|---|---|---|---|---|---|
| TPC | 1 | |||||
| TFC | 0.866 ** | 1 | ||||
| Proline | 0.882 ** | 0.971 ** | 1 | |||
| DPPH | −0.950 ** | −0.909 ** | −0.919 ** | 1 | ||
| FRAP | 0.962 ** | 0.860 ** | 0.857 ** | −0.971 ** | 1 | |
| Reducing Power | 0.954 ** | 0.915 ** | 0.916 ** | 0.963 ** | 0.969 ** | 1 |
| Count | Compound | RI | Rt [Sec] | Dt [RIPrel] | Peak Areas | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 7 | 9 | 12 | 14 | 16 | |||||
| Aldehydes | |||||||||||||
| 1 | nonanal | 1106.4 | 499.475 | 1.4738 | 166.43 | 172.19 | 177.55 | 186.49 | 163.34 | 167.27 | 182.64 | 236.66 | 226.20 |
| 2 | benzaldehyde-Mono | 955.9 | 306.492 | 1.1505 | 173.15 | 177.60 | 66.95 | 81.93 | 149.10 | 177.21 | 166.61 | 305.36 | 294.93 |
| 3 | benzaldehyde-Di | 954.5 | 305.332 | 1.4728 | 24.98 | 25.40 | 18.72 | 21.46 | 24.40 | 25.34 | 25.60 | 51.96 | 49.71 |
| 4 | heptanal | 892.2 | 260.223 | 1.3286 | 37.07 | 41.73 | 27.56 | 25.10 | 34.07 | 27.72 | 30.90 | 37.50 | 39.71 |
| 5 | pentanal | 687.9 | 166.099 | 1.1847 | 256.03 | 238.14 | 184.02 | 175.89 | 178.89 | 171.68 | 181.55 | 177.58 | 175.20 |
| 6 | hexanal-Mono | 784.4 | 203.044 | 1.2565 | 258.97 | 213.92 | 140.60 | 116.72 | 122.00 | 109.31 | 87.30 | 102.90 | 100.95 |
| 7 | hexanal-Di | 783.7 | 202.677 | 1.5647 | 194.96 | 135.51 | 74.13 | 59.96 | 61.79 | 56.98 | 45.52 | 52.88 | 56.47 |
| 8 | furfural | 820.5 | 220.276 | 1.0831 | 25.78 | 28.83 | 30.27 | 27.43 | 29.76 | 35.38 | 34.84 | 56.81 | 59.00 |
| Ketones | |||||||||||||
| 9 | 2-butanone-Mono | 568.1 | 138.666 | 1.0586 | 350.73 | 393.90 | 392.81 | 390.30 | 434.96 | 447.73 | 410.43 | 447.16 | 459.03 |
| 10 | 2-butanone-Di | 572.2 | 139.512 | 1.2468 | 650.94 | 766.18 | 1655.06 | 1657.30 | 1176.66 | 1056.55 | 945.86 | 919.30 | 950.37 |
| 11 | acetone | 486.1 | 121.76 | 1.1171 | 3860.92 | 3169.63 | 4247.45 | 4712.79 | 4203.60 | 3731.30 | 3861.29 | 3688.94 | 3768.52 |
| 12 | methyl isobutyl ketone-Mono | 739.8 | 183.982 | 1.1704 | 52.64 | 39.69 | 72.90 | 65.62 | 38.10 | 34.43 | 28.91 | 32.09 | 33.98 |
| 13 | methyl isobutyl ketone-Di | 738.3 | 183.368 | 1.4806 | 28.48 | 24.73 | 39.32 | 40.63 | 23.82 | 21.61 | 19.83 | 16.15 | 20.63 |
| 14 | methyl-5-hepten-2-one | 986 | 335.12 | 1.181 | 71.42 | 69.15 | 69.81 | 68.41 | 63.21 | 64.41 | 67.12 | 49.12 | 62.91 |
| 15 | 2-hexanone | 797 | 208.856 | 1.1858 | 92.45 | 94.66 | 94.18 | 94.67 | 108.63 | 110.44 | 107.73 | 114.47 | 127.39 |
| 16 | 2-pentanone | 674.6 | 162.378 | 1.1205 | 62.21 | 51.62 | 67.58 | 62.90 | 45.85 | 43.60 | 33.40 | 35.45 | 36.55 |
| Alcohols | |||||||||||||
| 17 | 3-octanol | 984.2 | 333.27 | 1.4009 | 77.04 | 71.69 | 49.22 | 47.13 | 34.98 | 43.37 | 36.43 | 27.59 | 35.12 |
| 18 | pentan-1-ol | 759.4 | 191.966 | 1.252 | 81.63 | 63.68 | 57.90 | 63.13 | 61.76 | 59.49 | 57.84 | 60.29 | 60.83 |
| 19 | 1-butanol-Mono | 653.9 | 157.1 | 1.18 | 150.56 | 143.49 | 101.96 | 97.48 | 97.30 | 93.71 | 87.95 | 84.51 | 81.34 |
| 20 | 1-butanol-Di | 644.6 | 154.886 | 1.3745 | 145.67 | 96.62 | 191.00 | 172.08 | 77.84 | 64.33 | 48.66 | 40.18 | 41.24 |
| 21 | ethanol | 442.5 | 112.756 | 1.0448 | 599.24 | 691.37 | 665.54 | 510.66 | 575.62 | 399.16 | 991.81 | 512.12 | 518.59 |
| Esters | |||||||||||||
| 22 | propyl butanoate | 887.7 | 257.468 | 1.2615 | 327.00 | 211.46 | 211.42 | 147.18 | 173.15 | 105.32 | 119.23 | 105.06 | 125.44 |
| 23 | n-propyl acetate | 709.7 | 172.973 | 1.1617 | 114.67 | 105.66 | 118.64 | 89.68 | 72.09 | 61.30 | 53.71 | 54.57 | 61.09 |
| 24 | ethyl acetate-Mono | 600.9 | 145.434 | 1.0965 | 127.95 | 99.31 | 76.45 | 73.50 | 82.71 | 93.08 | 89.40 | 86.09 | 90.59 |
| 25 | ethyl acetate-Di | 598.1 | 144.836 | 1.3381 | 42.49 | 26.59 | 18.19 | 15.61 | 20.97 | 25.38 | 23.06 | 23.30 | 22.51 |
| 26 | (Z)-3-hexen-1-ol-Di | 849.8 | 235.56 | 1.5036 | 7.69 | 14.28 | 18.74 | 25.13 | 28.33 | 69.31 | 36.43 | 40.32 | 48.46 |
| 27 | butyl acetate | 799.5 | 210.074 | 1.2371 | 69.23 | 46.52 | 32.97 | 31.44 | 31.72 | 26.79 | 27.48 | 26.21 | 29.40 |
| 28 | isopropyl acetate | 648.1 | 155.713 | 1.1573 | 29.98 | 31.83 | 21.21 | 24.16 | 30.23 | 36.10 | 32.61 | 41.06 | 38.14 |
| 29 | ethyl 2-methylbutyrate | 850.3 | 235.816 | 1.2316 | 17.47 | 20.44 | 28.35 | 31.81 | 34.72 | 58.52 | 38.34 | 49.37 | 49.89 |
| Acids | |||||||||||||
| 30 | 2-methylbutanoic acid-Di | 851.3 | 236.413 | 1.4727 | 124.68 | 170.58 | 179.85 | 203.49 | 213.06 | 215.23 | 205.71 | 197.46 | 212.03 |
| 31 | isovaleric acid | 839.9 | 230.272 | 1.2169 | 47.72 | 76.59 | 82.28 | 94.80 | 97.31 | 106.53 | 100.25 | 93.73 | 97.73 |
| 32 | acetic acid | 602.2 | 145.691 | 1.152 | 55.17 | 61.71 | 89.79 | 96.38 | 98.35 | 103.31 | 89.44 | 104.57 | 99.84 |
| Furan | |||||||||||||
| 33 | 2-ethyl furan | 684.5 | 165.125 | 1.3029 | 53.61 | 47.53 | 58.71 | 49.41 | 82.56 | 98.63 | 74.76 | 73.15 | 114.27 |
| Disulfide | |||||||||||||
| 34 | dimethyl disulfide | 752.4 | 189.027 | 1.1471 | 68.17 | 91.41 | 109.12 | 133.03 | 144.69 | 136.71 | 127.03 | 129.69 | 128.27 |
| Amine | |||||||||||||
| 35 | triethylamine | 686 | 165.546 | 1.2264 | 49.24 | 51.28 | 62.76 | 59.30 | 60.64 | 61.91 | 60.23 | 65.08 | 67.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.-Z.; Tian, J.; Zhang, Y.-Z.; Li, S.-S.; Zheng, H.-Q.; Hu, F.-L. Investigation of the Maturity Evaluation Indicator of Honey in Natural Ripening Process: The Case of Rape Honey. Foods 2021, 10, 2882. https://doi.org/10.3390/foods10112882
Zhang G-Z, Tian J, Zhang Y-Z, Li S-S, Zheng H-Q, Hu F-L. Investigation of the Maturity Evaluation Indicator of Honey in Natural Ripening Process: The Case of Rape Honey. Foods. 2021; 10(11):2882. https://doi.org/10.3390/foods10112882
Chicago/Turabian StyleZhang, Guo-Zhi, Jing Tian, Yan-Zheng Zhang, Shan-Shan Li, Huo-Qing Zheng, and Fu-Liang Hu. 2021. "Investigation of the Maturity Evaluation Indicator of Honey in Natural Ripening Process: The Case of Rape Honey" Foods 10, no. 11: 2882. https://doi.org/10.3390/foods10112882