Effects of Drying Process on the Volatile and Non-Volatile Flavor Compounds of Lentinula edodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
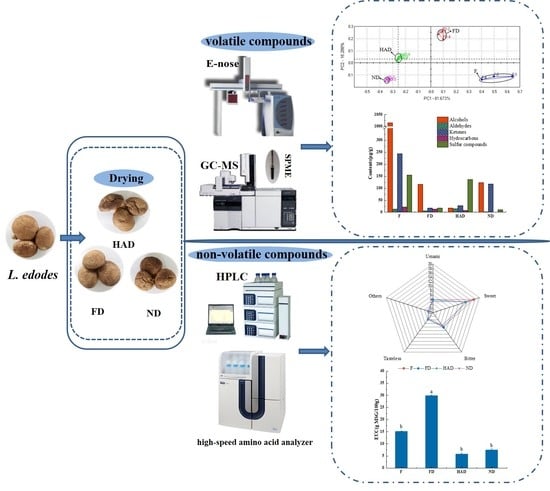
2.2. Drying Methods
2.2.1. Freeze-Drying (FD)
2.2.2. Hot Air Drying (HAD)
2.2.3. Natural Drying (ND)
2.3. Electronic Nose Analysis
2.4. HS-SPME-GC-MS Analysis
2.5. Assay of Organic Acids
2.6. Assay of Free Amino Acids
2.7. Assay of 5′-Nucleotides
2.8. Statistical Analysis
3. Results and Discussion
3.1. Electronic Nose Analysis of L. edodes Samples
3.2. HS-SPME-GC-MS Analysis of Volatile Compounds of L. edodes
3.3. Effects of Drying Methods on Organic Acids of L. edodes
3.4. Effects of Drying Methods on Free amino Acids of L. edodes
3.5. Effects of Drying Methods on 5′-Nucleotides of L. edodes
3.6. Equivalent Umami Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef]
- Mleczek, M.; Budka, A.; Siwulski, M.; Mleczek, P.; Gasecka, M.; Jasinska, A.; Kalac, P.; Sobieralski, K.; Niedzielski, P.; Proch, J.; et al. Investigation of differentiation of metal contents of Agaricus bisporus, Lentinula edodes and Pleurotus ostreatus sold commercially in Poland between 2009 and 2017. J. Food Compos. Anal. 2020, 90, 103488. [Google Scholar] [CrossRef]
- Gao, S.S.; Huang, Z.C.; Feng, X.; Bian, Y.B.; Huang, W.; Liu, Y. Bioconversion of rice straw agro-residues by Lentinula edodes and evaluation of non-volatile taste compounds in mushrooms. Sci. Rep. 2020, 10, 1814. [Google Scholar] [CrossRef]
- Reis, G.C.L.; Custodio, F.B.; Botelho, B.G.; Guidi, L.R.; Gloria, M.B.A. Investigation of biologically active amines in some selected edible mushrooms. J. Food Compos. Anal. 2020, 86, 103375. [Google Scholar] [CrossRef]
- Pil-Nam, S.; Park, K.-M.; Kang, G.-H.; Cho, S.-H.; Park, B.-Y.; Van-Ba, H. The impact of addition of shiitake on quality characteristics of frankfurter during refrigerated storage. LWT-Food Sci. Technol. 2015, 62, 62–68. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Hartung, N.M.; Fraatz, M.A.; Zorn, H. Quantification of key odor-active compounds of a novel nonalcoholic beverage produced by fermentation of wort by shiitake (Lentinula edodes) and aroma genesis studies. Food Res. Int. 2015, 70, 23–30. [Google Scholar] [CrossRef]
- Holighaus, G.; Weissbecker, B.; von Fragstein, M.; Schutz, S. Ubiquitous eight-carbon volatiles of fungi are infochemicals for a specialist fungivore. Chemoecology 2014, 24, 57–66. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, X.Y.; Chen, L.F.; Bian, Y.B.; Yang, H.; Ibrahim, S.A.; Huang, W. A novel cysteine desulfurase influencing organosulfur compounds in Lentinula edodes. Sci. Rep. 2015, 5, 10047. [Google Scholar] [CrossRef] [Green Version]
- Pei, F.; Yang, W.; Ma, N.; Fang, Y.; Zhao, L.; An, X.; Xin, Z.; Hu, Q. Effect of the two drying approaches on the volatile profiles of button mushroom (Agaricus bisporus) by headspace GC-MS and electronic nose. LWT-Food Sci. Technol. 2016, 72, 343–350. [Google Scholar] [CrossRef]
- Yang, W.; Yu, J.; Pei, F.; Mariga, A.M.; Ma, N.; Fang, Y.; Hu, Q. Effect of hot air drying on volatile compounds of Flammulina velutipes detected by HS-SPME-GC-MS and electronic nose. Food Chem. 2016, 196, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Feng, X.; Huang, W.; Ibrahim, S.A.; Liu, Y. Effects of drying methods on non-volatile taste components of Stropharia rugoso-annulata mushrooms. LWT-Food Sci. Technol. 2020, 127, 109428. [Google Scholar] [CrossRef]
- Politowicz, J.; Lech, K.; Lipan, L.; Figiel, A.; Carbonell-Barrachina, A.A. Volatile composition and sensory profile of shiitake mushrooms as affected by drying method. J. Sci. Food Agric. 2018, 98, 1511–1521. [Google Scholar] [CrossRef]
- Cheng, S.S.; Li, R.; Yang, H.M.; Wang, S.Q.; Lin, R.; Tan, M.Q. Characterisation of moisture migration of shiitake mushroom (Lentinula edodes) during storage and its relationship to quality deterioration. Int. J. Food Sci. Technol. 2020, 55, 2132–2140. [Google Scholar] [CrossRef]
- Lu, X.; Hou, H.; Fang, D.; Hu, Q.; Chen, J.; Zhao, L. Identification and characterization of volatile compounds in Lentinula edodes during vacuum freeze-drying. J. Food Biochem. 2021, e13814. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Gao, J.-X.; Xue, J.; Chen, D.; Lin, S.-Y.; Dong, X.-P.; Zhu, B.-W. Changes in Aroma Profile of Shiitake Mushroom (Lentinus edodes) during Different Stages of Hot Air Drying. Foods 2020, 9, 444. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Pan, S.; Fan, G.; Dong, L.; Ren, J.; Zhu, Y. Evaluation of volatile profile of Sichuan dongcai, a traditional salted vegetable, by SPME-GC-MS and E-nose. LWT-Food Sci. Technol. 2015, 64, 528–535. [Google Scholar] [CrossRef]
- Ren, J.N.; Tai, Y.N.; Dong, M.; Shao, J.H.; Yang, S.Z.; Pan, S.Y.; Fan, G. Characterisation of free and bound volatile compounds from six different varieties of citrus fruits. Food Chem. 2015, 185, 25–32. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Chrysanthou, A.; Koutidou, M. Characterisation of volatile compounds of lupin protein isolate-enriched wheat flour bread. Food Res. Int. 2012, 48, 568–577. [Google Scholar] [CrossRef]
- Li, X.; Feng, T.; Zhou, F.; Zhou, S.; Liu, Y.; Li, W.; Ye, R.; Yang, Y. Effects of drying methods on the tasty compounds of Pleurotus eryngii. Food Chem. 2015, 166, 358–364. [Google Scholar] [CrossRef]
- Hotel, O.; Poli, J.P.; Mer-Calfati, C.; Scorsone, E.; Saada, S. A review of algorithms for SAW sensors e-nose based volatile compound identification. Sens. Actuators B-Chem. 2018, 255, 2472–2482. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, J.; Zhang, Y.R.; Liu, Q.; Pan, L.Q.; Tu, K. Discrimination of volatiles of shiitakes (Lentinula edodes) produced during drying process by electronic nose. Int. J. Food Eng. 2020, 16, 20190233. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Li, D.; Wang, M. Electronic Tongue Coupled with Physicochemical Analysis for the Recognition of Orange Beverages. J. Food Qual. 2012, 35, 429–441. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, D.-J.; Kim, J.-Y.; Lim, S.-T. Volatile composition and sensory characteristics of onion powders prepared by convective drying. Food Chem. 2017, 231, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, G.; Shanmugam, S.; Galvao, M.d.S.; Dutra Sandes, R.D.; Santos Leite Neta, M.T.; Narain, N.; Mujumdar, A.S. Comparative evaluation of physical properties and volatiles profile of cabbages subjected to hot air and freeze drying. LWT-Food Sci. Technol. 2017, 80, 501–509. [Google Scholar] [CrossRef]
- Tan, H.R.; Lau, H.; Liu, S.Q.; Tan, L.P.; Sakumoto, S.; Lassabliere, B.; Leong, K.-C.; Sun, J.; Yu, B. Characterisation of key odourants in Japanese green tea using gas chromatography-olfactometry and gas chromatography-mass spectrometry. LWT-Food Sci. Technol. 2019, 108, 221–232. [Google Scholar] [CrossRef]
- Shi, Y.; Li, X.; Huang, A. A metabolomics-based approach investigates volatile flavor formation and characteristic compounds of the Dahe black pig dry-cured ham. Meat Sci. 2019, 158, 107904. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Cao, J.; Zou, X.-G.; Deng, L.; Fan, Y.-W.; Li, H.; Li, J.; Deng, Z.-Y. Analysis of nonpolar lipophilic aldehydes/ketones in oxidized edible oils using HPLC-QqQ-MS for the evaluation of their parent fatty acids. Food Res. Int. 2014, 64, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, J.; Ye, X.; Lv, B.; Chu, Y.; Chen, J. Advances on flavor substances of edible mushrooms. Sci. Technol. Food Ind. 2012, 33, 412–414. [Google Scholar]
- Jung, M.Y.; Lee, D.E.; Baek, S.H.; Lim, S.M.; Chung, I.-M.; Han, J.-G.; Kim, S.-H. An unattended HS-SPME-GC-MS/MS combined with a novel sample preparation strategy for the reliable quantitation of C8 volatiles in mushrooms: A sample preparation strategy to fully control the volatile emission. Food Chem. 2021, 347, 128998. [Google Scholar] [CrossRef]
- Combet, E.; Henderson, J.; Eastwood, D.C.; Burton, K.S. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Corral, S.; Leitner, E.; Siegmund, B.; Flores, M. Determination of sulfur and nitrogen compounds during the processing of dry fermented sausages and their relation to amino acid generation. Food Chem. 2016, 190, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, Y.; Toyoda, M.; Suzuki, H.; Iwaida, M. Gas-liquid-chromatographic determination of lenthionine in shiitake mushroom (lentinus-edodes) with special reference to relation between carbon-disulfide and lenthionine. J. Food Sci. 1978, 43, 1287–1289. [Google Scholar] [CrossRef]
- Hiraide, M.; Miyazaki, Y.; Shibata, Y. The smell and odorous components of dried shiitake mushroom, Lentinula edodes I: Relationship between sensory evaluations and amounts of odorous components. J. Wood Sci. 2004, 50, 358–364. [Google Scholar] [CrossRef]
- Chen, C.C.; Wu, C.M. Studies on the enzymic reduction of 1-octen-3-one in mushroom (agaricus-bisporus). J. Agric. Food Chem. 1984, 32, 1342–1344. [Google Scholar] [CrossRef]
- Chen, W.; Li, W.; Yang, Y.; Yu, H.; Zhou, S.; Feng, J.; Li, X.; Liu, Y. Analysis and Evaluation of Tasty Components in the Pileus and Stipe of Lentinula edodes at Different Growth Stages. J. Agric. Food Chem. 2015, 63, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Yang, W.-J.; Shi, Y.; Sun, Y.; Mariga, A.M.; Zhao, L.-Y.; Fang, Y.; Ma, N.; An, X.-X.; Hu, Q.-H. Comparison of Freeze-Drying with Three Different Combinations of Drying Methods and Their Influence on Colour, Texture, Microstructure and Nutrient Retention of Button Mushroom (Agaricus bisporus) Slices. Food Bioprocess Technol. 2014, 7, 702–710. [Google Scholar] [CrossRef]
- Xu, L.; Fang, X.; Wu, W.; Chen, H.; Mu, H.; Gao, H. Effects of high-temperature pre-drying on the quality of air-dried shiitake mushrooms (Lentinula edodes). Food Chem. 2019, 285, 406–413. [Google Scholar] [CrossRef]
- Yin, C.; Fan, X.; Fan, Z.; Shi, D.; Yao, F.; Gao, H. Comparison of non-volatile and volatile flavor compounds in six Pleurotus mushrooms. J. Sci. Food Agric. 2019, 99, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Lin, H.C.; Mau, J.L. Non-volatile taste components of several commercial mushrooms. Food Chem. 2001, 72, 465–471. [Google Scholar] [CrossRef]




| Compounds | RI a | Contents (μg/g) | ID e | ||||
|---|---|---|---|---|---|---|---|
| F b | FD | HAD | ND | ||||
| Alcohols (11) | 1-Hexanol | 871 | nd d | 0.40 | 0.56 | 1.17 | A |
| 1-Octen-3-ol c | 983 | 857.02 | 103.78 | 5.72 | 105.30 | B | |
| 2-Cyclohexen-1-ol | 985 | nd | nd | 7.22 | nd | A | |
| 3-Octanol c | 998 | 6.09 | 1.93 | nd | 1.40 | B | |
| 2-Octen-1-ol c | 1071 | 132.99 | 6.35 | 2.59 | 13.86 | B | |
| 1-Octanol c | 1077 | 19.89 | 1.99 | 0.68 | 2.06 | B | |
| Linalool | 1100 | nd | 2.13 | nd | nd | A | |
| Phenylethyl Alcohol | 1117 | nd | 0.08 | 0.95 | nd | A | |
| 4-Methyl-benzeneethanol | 1173 | nd | nd | 0.66 | nd | A | |
| α-Terpineol | 1188 | nd | 0.43 | nd | nd | B | |
| Cedrol | 1596 | nd | 0.11 | nd | nd | B | |
| Total alcohols | 1015.99 | 117.20 | 18.39 | 123.79 | |||
| Aldehydes (9) | Hexanal | 803 | nd | 0.34 | 4.62 | 0.02 | A |
| (E)-Hept-2-enal | 958 | nd | nd | nd | 0.18 | A | |
| Benzaldehyde | 965 | 0.69 | 0.62 | 1.30 | 0.15 | A | |
| Octanal c | 1004 | nd | nd | 0.18 | nd | A | |
| Benzeneacetaldehyde | 1046 | 0.48 | 3.44 | 0.93 | 0.32 | A | |
| (E)-2-Octenal c | 1058 | 11.78 | nd | 1.76 | 1.72 | A | |
| 2-Phenylpropenal | 1157 | nd | 0.67 | 5.64 | 0.07 | A | |
| Decanal | 1206 | 0.69 | 0.42 | 0.27 | 0.09 | A | |
| (z)-3,7-Dimethylocta-2,6-dienal | 1241 | nd | 1.18 | nd | nd | A | |
| Total aldehydes | 13.64 | 6.67 | 14.70 | 2.55 | |||
| Ketones (6) | 1-Octen-3-one c | 981 | 237.99 | 5.52 | 21.17 | 118.24 | B |
| 3-Octanone c | 991 | 6.09 | 11.61 | 6.79 | nd | B | |
| 3-Octen-2-one c | 1042 | nd | nd | nd | 0.11 | A | |
| Acetophenone | 1066 | nd | nd | 0.14 | 0.02 | A | |
| (+)-Camphor | 1139 | 0.18 | 0.08 | 0.05 | 0.02 | B | |
| 2-Undecanone | 1297 | nd | 0.06 | 0.34 | 0.01 | A | |
| Total ketones | 244.26 | 17.27 | 28.49 | 118.40 | |||
| Sulfur compounds (11) | Carbon disulfide | <700 | 148.36 | 12.51 | 66.31 | 11.01 | A |
| Dimethyl disulfide | 700 | nd | nd | 2.58 | nd | B | |
| Dimethyl trisulfide | 973 | nd | 0.80 | 0.26 | nd | B | |
| 1,2,4-Trithiolane | 1083 | 7.00 | 2.79 | 32.21 | nd | B | |
| 2,4,5-Trithiahexane | 1121 | nd | nd | 4.87 | nd | A | |
| Dimethyl tetrasulfide | 1209 | nd | nd | 2.27 | nd | A | |
| 1,2,4,5-Tetrathiane | 1362 | nd | 0.11 | 7.23 | nd | B | |
| 1,2,4,6-Tetrathiepane | 1504 | nd | 0.01 | 3.22 | nd | B | |
| Lenthionine | 1615 | 0.24 | 1.00 | 17.11 | 0.04 | B | |
| Hexathiepane | 1615 | nd | nd | 0.25 | nd | B | |
| Cyclic octaatomic sulfur | 2000 | nd | nd | 0.29 | nd | A | |
| Total sulfur compounds | 155.60 | 17.22 | 136.60 | 11.05 | |||
| Hydrocarbons (17) | 1,3-Xylene | 865 | nd | 0.05 | 0.04 | 0.03 | A |
| P-Isopropyltoluene | 1021 | 1.06 | 0.32 | nd | 0.03 | A | |
| D-Limonene | 1025 | 17.43 | 10.54 | 7.74 | 0.78 | A | |
| Naphthalene | 1176 | 0.30 | 0.33 | 0.54 | 0.02 | A | |
| Dodecane | 1199 | 0.16 | 0.38 | nd | nd | A | |
| 5-Ethyl-2-methyl-octane | 1280 | 0.95 | nd | nd | nd | A | |
| 3-Carene | 1283 | nd | 0.07 | nd | nd | B | |
| Decane | 1288 | nd | 0.13 | nd | nd | A | |
| b-Elemen | 1390 | nd | 0.08 | nd | nd | A | |
| Tetradecane | 1400 | 0.56 | 0.14 | 0.09 | 0.00 | A | |
| Germacrene D | 1477 | nd | 0.08 | nd | nd | A | |
| (-)-a-Selinenea- | 1491 | nd | 0.20 | nd | nd | A | |
| Pentadecane | 1501 | 0.91 | 0.39 | nd | nd | A | |
| Hexadecane | 1600 | nd | 0.11 | 0.05 | 0.02 | A | |
| Heptadecane | 1697 | nd | 0.05 | nd | nd | A | |
| Eicosane | 2000 | 0.14 | 0.06 | nd | nd | A | |
| Total hydrocarbons | 21.51 | 12.93 | 8.46 | 0.88 | |||
| Organic Acids (mg/g) | F a | FD | HAD | ND |
|---|---|---|---|---|
| Tartaric acid | 41.91 ± 1.97 A | Nd b | 12.18 ± 0.19 B | Nd b |
| Malic acid | 36.22 ± 0.70 A | 27.82 ± 0.77 B | 23.57 ± 2.35 BC | 21.00 ± 0.38 C |
| Ascorbic acid | 6.27 ± 0.28 A | 5.00 ± 0.27 C | 5.66 ± 0.11 B | 4.88 ± 0.18 C |
| Citric acid | 134.49 ± 0.45 C | 107.65 ± 2.17 D | 138.13 ± 1.90 B | 142.31 ± 1.09 A |
| Fumaric acid | 11.68 ± 0.03 A | 8.23 ± 0.03 B | 6.57 ± 0.02 D | 7.61 ± 0.02 C |
| Succinic acid | 645.25 ± 3.03 A | 472.61 ± 3.34 D | 481.71 ± 1.72 C | 519.51 ± 2.11 B |
| Total | 875.82 ± 2.04 A | 621.32 ± 3.42 D | 667.82 ± 0.48 C | 695.30 ± 0.96 B |
| Free Amino Acids (mg/g) | F a | FD | HAD | ND | |
|---|---|---|---|---|---|
| Umami Taste Amino Acids | Asp b | 0.56 ± 0.01 B | 1.57 ± 0.001 A | 0.10 ± 0.01 C | 0.10 ± 0.001 C |
| Glu | 3.33 ± 0.02 A | 2.40 ± 0.001 C | 3.26 ± 0.01 A | 2.76 ± 0.003 B | |
| total | 3.89 ± 0.02 AB | 3.97 ± 0.001 A | 3.36 ± 0.02 AB | 2.86 ± 0.003 B | |
| Ala | 0.74 ± 0.003 D | 1.27 ± 0.003 B | 1.80 ± 0.01 A | 0.95 ± 0.003 C | |
| Sweet Taste Amino Acids | Gly | 0.77 ± 0.003 A | 0.85 ± 0.001 A | 0.80 ± 0.01 A | 0.57 ± 0.003 B |
| Ser | 0.76 ± 0.01 A | 0.72 ± 0.003 A | 0.46 ± 0.003 B | 0.39 ± 0.003 C | |
| Thr | 14.89 ± 0.01 A | 10.57 ± 0.05 B | 10.59 ± 0.07 AB | 13.10 ± 0.08 A | |
| total | 17.16 ± 0.02 A | 13.41 ± 0.06 B | 13.65 ± 0.08 AB | 15.00 ± 0.07 A | |
| Arg | 2.77 ± 0.002 A | 2.07 ± 0.003 C | 1.51 ± 0.01 C | 2.45 ± 0.01 B | |
| Bitter Taste Amino Acids | His | 0.68 ± 0.001 A | 0.55 ± 0.001 B | 0.37 ± 0.01 C | 0.36 ± 0.001 C |
| Ile | 0.11 ± 0.001 B | 0.43 ± 0.001 A | 0.42 ± 0.001 A | 0.15 ± 0.001 B | |
| Leu | 0.20 ± 0.001 B | 0.71 ± 0.001 A | 0.66 ± 0.01 AB | 0.14 ± 0.003 B | |
| Met | 0.10 ± 0.001 A | 0.08 ± 0.01 A | 0.07 ± 0.003 A | 0.08 ± 0.01 A | |
| Phe | 0.67 ± 0.09 A | 0.79 ± 0.01 A | 0.92 ± 0.03 A | 0.56 ± 0.01 B | |
| Val | 1.33 ± 0.01 B | 1.64 ± 0.003 A | 1.36 ± 0.01 AB | 1.10 ± 0.003 C | |
| total | 5.85 ± 0.10 AB | 6.28 ± 0.02 A | 5.30 ± 0.07 AB | 4.83 ± 0.02 B | |
| Tasteless Amino Acids | Lys | 1.16 ± 0.003 B | 1.43 ± 0.003 A | 1.17 ± 0.001 B | 0.86 ± 0.003 C |
| Tyr | 0.28 ± 0.01 A | 0.34 ± 0.003 A | 0.42 ± 0.03 A | 0.18 ± 0.02 A | |
| total | 1.44 ± 0.02 B | 1.77 ± 0.001 A | 1.59 ± 0.03 B | 1.04 ± 0.02 B | |
| Others | GABA | 0.20 ± 0.001 B | 0.13 ± 0.003 B | 0.41 ± 0.003 A | 0.51 ± 0.001 A |
| Orn | 4.29 ± 0.01 C | 3.57 ± 0.01 D | 5.32 ± 0.01 B | 6.25 ± 0.01 A | |
| Total Amino Acids | 32.81 ± 0.09 A | 29.13 ± 0.06 C | 29.61 ± 0.08 BC | 30.48 ± 0.03 B | |
| 5′-Nucleotides a | F b | FD | HAD | ND |
|---|---|---|---|---|
| 5′-CMP | 3.14 ± 0.03 B | 2.96 ± 0.03 B | 7.78 ± 0.03 A | 8.38 ± 0.06 A |
| 5′-UMP | 0.27 ± 0.02 C | 0.39 ± 0.004 B | 0.92 ± 0.03 B | 1.92 ± 0.09 A |
| 5′-GMP | 0.33 ± 0.004 A | 0.22 ± 0.003 A | 0.39 ± 0.03 A | 0.57 ± 0.03 A |
| 5′-AMP | 4.25 ± 0.01 A | 4.92 ± 0.02 A | 2.60 ± 0.01 B | 3.69 ± 0.26 B |
| Flavor 5′-nucleotides c | 0.33 ± 0.004 A | 0.22 ± 0.003 A | 0.39 ± 0.03 A | 0.57 ± 0.03 A |
| MSG-like 5′-nucleotide d | 4.58 ± 0.002 A | 5.14 ± 0.02 A | 2.99 ± 0.02 B | 4.26 ± 0.29 A |
| Total | 7.94 ± 0.05 B | 8.48 ± 0.00 B | 11.61 ± 0.08 A | 14.41 ± 0.14 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Dong, X.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Effects of Drying Process on the Volatile and Non-Volatile Flavor Compounds of Lentinula edodes. Foods 2021, 10, 2836. https://doi.org/10.3390/foods10112836
Zhang L, Dong X, Feng X, Ibrahim SA, Huang W, Liu Y. Effects of Drying Process on the Volatile and Non-Volatile Flavor Compounds of Lentinula edodes. Foods. 2021; 10(11):2836. https://doi.org/10.3390/foods10112836
Chicago/Turabian StyleZhang, Lijia, Xiaobo Dong, Xi Feng, Salam A. Ibrahim, Wen Huang, and Ying Liu. 2021. "Effects of Drying Process on the Volatile and Non-Volatile Flavor Compounds of Lentinula edodes" Foods 10, no. 11: 2836. https://doi.org/10.3390/foods10112836