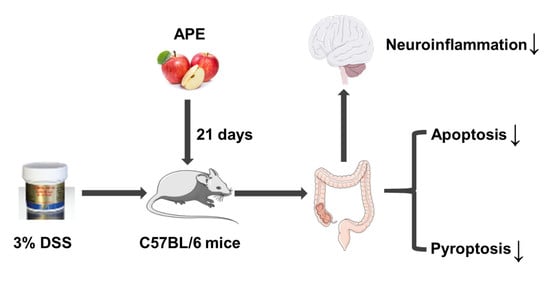
Apple Polyphenols Extract (APE) Alleviated Dextran Sulfate Sodium Induced Acute Ulcerative Colitis and Accompanying Neuroinflammation via Inhibition of Apoptosis and Pyroptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Histological Analyses of Colon Tissue
2.4. Analysis of Serum Samples
2.5. RT-PCR Analysis
2.6. Immunohistochemistry
2.7. Alcian Blue/Periodic Acid-Schiff (AB-PAS) Staining
2.8. Western Blot Analysis
2.9. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling Assay (TUNEL)
2.10. Statistical Analysis
3. Results
3.1. Effect of APE on the Symptoms of DSS-Induced Acute UC
3.2. Effect of APE on Pro-Inflammatory Mediators
3.3. Effect of APE on Intestinal Tight Junction Proteins
3.4. Effect of APE on the Function of Goblet Cells
3.5. Effect of APE on IEC Apoptosis
3.6. Effect of APE on the Pyroptosis Signaling Pathway
3.7. Effect of APE on Acute UC-Related Neuroinflammation and Synapse Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, P.D.; Zhao, Y.H. Targeting NF-κB pathway for treating ulcerative colitis: Comprehensive regulatory characteristics of Chinese medicines. Chin. Med. 2020, 15, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Z.; Zhu, Z.; Yang, Y.; Ruan, W.; Peng, X.; Su, Y.; Peng, L.; Chen, J.; Yin, Q.; Zhao, C.; et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: A prospective population-based study. J. Gastroenterol. Hepatol. 2013, 28, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Park, J.; Cheon, J.H. Incidence and Prevalence of Inflammatory Bowel Disease across Asia. Yonsei Med. J. 2021, 62, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ng, S.C.; Lei, Y.; Yi, F.; Li, J.; Yu, L.; Zou, K.; Dan, Z.; Dai, M.; Ding, Y.; et al. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: The emergence of “western” disease. Inflamm. Bowel Dis. 2013, 19, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis.-A-Mon. 2019, 65, 100851. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Benchimol, E.I.; Bitton, A.; Murthy, S.K.; Nguyen, G.C.; Lee, K.; Cooke-Lauder, J.; Kaplan, G.G. The Impact of Inflammatory Bowel Disease in Canada 2018: Extra-intestinal Diseases in IBD. J. Can. Assoc. Gastroenterol. 2019, 2, S73–S80. [Google Scholar] [CrossRef] [Green Version]
- Snapper, S.B.; Syngal, S.; Friedman, L.S. Ulcerative colitis and colon cancer: More controversy than clarity. Dig. Dis. 1998, 16, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Duprez, L.; Wirawan, E.; Vanden Berghe, T.; Vandenabeele, P. Major cell death pathways at a glance. Microbes Infect. 2009, 11, 1050–1062. [Google Scholar] [CrossRef]
- Dirisina, R.; Katzman, R.B.; Goretsky, T.; Managlia, E.; Mittal, N.; Williams, D.B.; Qiu, W.; Yu, J.; Chandel, N.S.; Zhang, L.; et al. p53 and PUMA independently regulate apoptosis of intestinal epithelial cells in patients and mice with colitis. Gastroenterology 2011, 141, 1036–1045. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cui, X.; Zhu, C.; Li, M.; Zhao, J.; Shen, Z.; Shan, X.; Wang, L.; Wu, H.; Shen, Y.; et al. FKBP11 protects intestinal epithelial cells against inflammation-induced apoptosis via the JNK-caspase pathway in Crohn’s disease. Mol. Med. Rep. 2018, 18, 4428–4438. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Zhu, L.; Liu, Y.; Zhang, L.; Liu, J.; Shen, H. Protective effects of paeoniflorin on TNBS-induced ulcerative colitis through inhibiting NF-kappaB pathway and apoptosis in mice. Int. Immunopharmacol. 2017, 50, 152–160. [Google Scholar] [CrossRef]
- Chae, J.M.; Chang, M.H.; Heo, W.; Cho, H.T.; Lee, D.H.; Hwang, B.B.; Kim, J.W.; Yoon, S.M.; Yang, S.; Lee, J.H.; et al. LB-9, Novel Probiotic Lactic Acid Bacteria, Ameliorates Dextran Sodium Sulfate-Induced Colitis in Mice by Inhibiting TNF-α-Mediated Apoptosis of Intestinal Epithelial Cells. J. Med. Food 2019, 22, 271–276. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Shen, W.; Wang, Y.; Cao, Y.; Nuerbulati, N.; Chen, W.; Lu, G.; Xiao, W.; Qi, R. Grape Seed Polyphenols Ameliorated Dextran Sulfate Sodium-Induced Colitis via Suppression of Inflammation and Apoptosis. Pharmacology 2020, 105, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zhaolin, Z.; Guohua, L.; Shiyuan, W.; Zuo, W. Role of pyroptosis in cardiovascular disease. Cell Prolif. 2019, 52, e12563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, C.A.; Mitchell, W.J.; Dadelahi, A.S.; Skyberg, J.A. Caspase-1 and Caspase-11 Mediate Pyroptosis, Inflammation, and Control of Brucella Joint Infection. Infect. Immun. 2018, 86, e00381-18. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Bu, R.; Yang, Q.; Jia, J.; Li, T.; Wang, Q.; Chen, Y. Exendin-4 Protects against Hyperglycemia-Induced Cardiomyocyte Pyroptosis via the AMPK-TXNIP Pathway. J. Diabetes Res. 2019, 2019, 8905917. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Nalbantoglu, I.; Ortega-Fernandez, S.; Aitken, J.D.; Su, Y.; Koren, O.; Walters, W.A.; Knight, R.; Ley, R.E.; Vijay-Kumar, M.; et al. Interleukin-1β (IL-1β) promotes susceptibility of Toll-like receptor 5 (TLR5) deficient mice to colitis. Gut 2012, 61, 373–384. [Google Scholar] [CrossRef]
- Nowarski, R.; Jackson, R.; Gagliani, N.; de Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef] [Green Version]
- Jie, F.; Xiao, S.; Qiao, Y.; You, Y.; Feng, Y.; Long, Y.; Li, S.; Wu, Y.; Li, Y.; Du, Q. Kuijieling decoction suppresses NLRP3-Mediated pyroptosis to alleviate inflammation and experimental colitis in vivo and in vitro. J. Ethnopharmacol. 2021, 264, 113243. [Google Scholar] [CrossRef] [PubMed]
- Demon, D.; Kuchmiy, A.; Fossoul, A.; Zhu, Q.; Kanneganti, T.D.; Lamkanfi, M. Caspase-11 is expressed in the colonic mucosa and protects against dextran sodium sulfate-induced colitis. Mucosal Immunol. 2014, 7, 1480–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, I.C.; TeKippe, E.M.; Woodford, R.M.; Uronis, J.M.; Holl, E.K.; Rogers, A.B.; Herfarth, H.H.; Jobin, C.; Ting, J.P. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 2010, 207, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Do, J.; Woo, J. From Gut to Brain: Alteration in Inflammation Markers in the Brain of Dextran Sodium Sulfate-induced Colitis Model Mice. Clin. Psychopharmacol. Neurosci. 2018, 16, 422–433. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut-Brain Axis Balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.C.; Furtos, A.; Dudonné, S.; Montoudis, A.; Garofalo, C.; Desjardins, Y.; Delvin, E.; Levy, E. Apple peel polyphenols and their beneficial actions on oxidative stress and inflammation. PLoS ONE 2013, 8, e53725. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.T.; Tu, S.H.; Yang, P.S.; Hsu, S.P.; Lee, W.H.; Ho, C.T.; Wu, C.H.; Lai, Y.H.; Chen, M.Y.; Chen, L.C. Apple Polyphenol Phloretin Inhibits Colorectal Cancer Cell Growth via Inhibition of the Type 2 Glucose Transporter and Activation of p53-Mediated Signaling. J. Agric. Food Chem. 2016, 64, 6826–6837. [Google Scholar] [CrossRef]
- Yeganeh, P.R.; Leahy, J.; Spahis, S.; Patey, N.; Desjardins, Y.; Roy, D.; Delvin, E.; Garofalo, C.; Leduc-Gaudet, J.P.; St-Pierre, D.; et al. Apple peel polyphenols reduce mitochondrial dysfunction in mice with DSS-induced ulcerative colitis. J. Nutr. Biochem. 2018, 57, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X.; Li, D.; Cui, Y.; Li, X. Apple polyphenols extract alleviated dextran sulfate sodium-induced ulcerative colitis in C57BL/6 male mice by restoring bile acid metabolism disorder and gut microbiota dysbiosis. Phytother. Res. 2020, 35, 1468–1485. [Google Scholar] [CrossRef]
- Denis, M.C.; Roy, D.; Yeganeh, P.R.; Desjardins, Y.; Varin, T.; Haddad, N.; Amre, D.; Sané, A.T.; Garofalo, C.; Furtos, A.; et al. Apple peel polyphenols: A key player in the prevention and treatment of experimental inflammatory bowel disease. Clin. Sci. 2016, 130, 2217–2237. [Google Scholar] [CrossRef] [Green Version]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef] [Green Version]
- Shastri, M.D.; Chong, W.C.; Vemuri, R.; Martoni, C.J.; Adhikari, S.; Bhullar, H.; Kunde, D.; Tristram, S.G.; Eri, R.D. Streptococcus Thermophilus UASt-09 Upregulates Goblet Cell Activity in Colonic Epithelial Cells to a Greater Degree than other Probiotic Strains. Microorganisms 2020, 8, 1758. [Google Scholar] [CrossRef]
- Subramanian, S.; Geng, H.; Tan, X.D. Cell death of intestinal epithelial cells in intestinal diseases. Sheng Li Xue Bao (Acta Physiol. Sin.) 2020, 72, 308–324. [Google Scholar]
- Armacki, M.; Trugenberger, A.K.; Ellwanger, A.K.; Eiseler, T.; Schwerdt, C.; Bettac, L.; Langgartner, D.; Azoitei, N.; Halbgebauer, R.; Groß, R.; et al. Thirty-eight-negative kinase 1 mediates trauma-induced intestinal injury and multi-organ failure. J. Clin. Investig. 2018, 128, 5056–5072. [Google Scholar] [CrossRef] [PubMed]
- Boise, L.H.; Gottschalk, A.R.; Quintáns, J.; Thompson, C.B. Bcl-2 and Bcl-2-related proteins in apoptosis regulation. Curr. Top. Microbiol. Immunol. 1995, 200, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, G.S.; Al-Harbi, S.; Almasan, A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol. Biol. 2015, 1219, 1–9. [Google Scholar] [CrossRef]
- Yao, J.; Cao, X.; Zhang, R.; Li, Y.X.; Xu, Z.L.; Zhang, D.G.; Wang, L.S.; Wang, J.Y. Protective Effect of Baicalin Against Experimental Colitis via Suppression of Oxidant Stress and Apoptosis. Pharmacogn. Mag. 2016, 12, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stutz, A.; Kolbe, C.C.; Stahl, R.; Horvath, G.L.; Franklin, B.S.; van Ray, O.; Brinkschulte, R.; Geyer, M.; Meissner, F.; Latz, E. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J. Exp. Med. 2017, 214, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhao, S.; Zhou, J.; Yan, J.; Wang, L.; Du, X.; Li, H.; Chen, Y.; Cai, W.; Wu, J. Curcumin alleviates DSS-induced colitis via inhibiting NLRP3 inflammsome activation and IL-1β production. Mol. Immunol. 2018, 104, 11–19. [Google Scholar] [CrossRef]
- Mai, C.T.; Wu, M.M.; Wang, C.L.; Su, Z.R.; Cheng, Y.Y.; Zhang, X.J. Palmatine attenuated dextran sulfate sodium (DSS)-induced colitis via promoting mitophagy-mediated NLRP3 inflammasome inactivation. Mol. Immunol. 2019, 105, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, X.; Zhang, X.; Tang, J.; Li, Z.; Wang, Q.; Hu, R. Oroxylin A inhibits colitis by inactivating NLRP3 inflammasome. Oncotarget 2017, 8, 58903–58917. [Google Scholar] [CrossRef]
- Chao, L.; Li, Z.; Zhou, J.; Chen, W.; Li, Y.; Lv, W.; Guo, A.; Qu, Q.; Guo, S. Shen-Ling-Bai-Zhu-San Improves Dextran Sodium Sulfate-Induced Colitis by Inhibiting Caspase-1/Caspase-11-Mediated Pyroptosis. Front. Pharmacol. 2020, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Cheng, X.; Zhang, J.; Yuan, C.; Zhao, M.; Yang, X. Ethyl pyruvate confers protection against endotoxemia and sepsis by inhibiting caspase-11-dependent cell pyroptosis. Int. Immunopharmacol. 2020, 78, 106016. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Abautret-Daly, Á.; Dempsey, E.; Parra-Blanco, A.; Medina, C.; Harkin, A. Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr. 2018, 30, 275–296. [Google Scholar] [CrossRef] [Green Version]
- Addolorato, G.; Capristo, E.; Stefanini, G.F.; Gasbarrini, G. Inflammatory bowel disease: A study of the association between anxiety and depression, physical morbidity, and nutritional status. Scand. J. Gastroenterol. 1997, 32, 1013–1021. [Google Scholar] [CrossRef]
- Felger, J.C. Role of Inflammation in Depression and Treatment Implications. Handb. Exp. Pharmacol. 2019, 250, 255–286. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Cao, H.; Shen, P.; Liu, J.; Fu, Y.; Cao, Y.; Zhang, N. The protective role of phloretin against dextran sulfate sodium-induced ulcerative colitis in mice. Food Funct. 2019, 10, 422–431. [Google Scholar] [CrossRef]









| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Il-1β | GCGTTGGCCCTCGGTCGAGTTCTA | CGGTCATATAGGAGCCCTTG |
| Il-18 | GACTCTTGCGTCAACTTCAAGG | CAGGCTGTCTTTTGTCAACGA |
| Zo-1 | ACCACCAACCCGAGAAGAC | CAGGAGTCATGGACGCACA |
| Occludin | TTGAAAGTCCACCTCCTTACAGA | CCGGATAAAAAGAGTACGCTGG |
| Gfap | TCTATGAGGAGGAAGTTCGAGA | TGCAAACTTAGACCGATACCA |
| Cox-2 | CAGACAACATAAACTGCGCCTT | GATACACCTCTCCACCAATGACC |
| Bdnf | CTCCGCCATGCAATTTCCACT | GCCTTCATGCAACCGAAGTA |
| Psd-95 | TCTGTGCGAGAGGTAGCAGA | AAGCACTCCGTGAACTCCTG |
| Gapdh | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Wang, X.; Cui, Y.; Yin, Y.; Qiu, D.; Li, S.; Li, X. Apple Polyphenols Extract (APE) Alleviated Dextran Sulfate Sodium Induced Acute Ulcerative Colitis and Accompanying Neuroinflammation via Inhibition of Apoptosis and Pyroptosis. Foods 2021, 10, 2711. https://doi.org/10.3390/foods10112711
Liu F, Wang X, Cui Y, Yin Y, Qiu D, Li S, Li X. Apple Polyphenols Extract (APE) Alleviated Dextran Sulfate Sodium Induced Acute Ulcerative Colitis and Accompanying Neuroinflammation via Inhibition of Apoptosis and Pyroptosis. Foods. 2021; 10(11):2711. https://doi.org/10.3390/foods10112711
Chicago/Turabian StyleLiu, Fang, Xinjing Wang, Yuan Cui, Yan Yin, Dong Qiu, Shilan Li, and Xinli Li. 2021. "Apple Polyphenols Extract (APE) Alleviated Dextran Sulfate Sodium Induced Acute Ulcerative Colitis and Accompanying Neuroinflammation via Inhibition of Apoptosis and Pyroptosis" Foods 10, no. 11: 2711. https://doi.org/10.3390/foods10112711