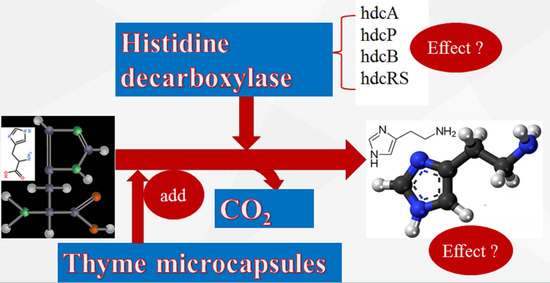
Impact of Thyme Microcapsules on Histamine Production by Proteus bacillus in Xinjiang Smoked Horsemeat Sausage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
2.2. Determination of Relevant Indicators in a Pure Culture System
2.3. Sausage Sample Preparation
2.4. Microbial Counts
2.5. RNA Extraction
2.6. Assessment of RNA Levels by RT-qPCR
2.7. Histamine Determination from Sausage Samples
2.8. Sensory Evaluation
2.9. Statistical Analyses
3. Results and Discussion
3.1. The Thyme Microcapsules Effect on P. bacillus Growth and Histamine Generation
3.2. Gene Expression in Pure Culture
3.3. Microbiological Analyses of Smoked Horsemeat Sausages
3.4. Gene Expression of hdcA and hdcP in Smoked Horsemeat Sausages
3.5. Histamine Accumulation in Smoked Horsemeat Sausages
3.6. Sensory Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ladero, V.; Calles-Enríquez, M.; Fernández, M.; Álvarez, M.A. Toxicological effects of dietary biogenic amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Linares, D.M.; Del Río, B.; Ladero, V.; Martínez, N.; Fernández, M.; Martín, M.C.; Álvarez, M.A. Factors influencing biogenic amines accumulation in dairy products. Front. Microbiol. 2012, 3, 180. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Xue, L.; Li, B.; Wang, Q.; Li, B.; Lu, S.; Fan, Q. Ferulic acid reduced histamine levels in the smoked horsemeat sausage. Int. J. Food Sci. Tech. 2018, 53, 2256–2264. [Google Scholar] [CrossRef]
- Lin, C.; Tsai, H.; Lin, C.; Huang, C.; Kung, H.; Tsai, Y. Histamine content and histamine-forming bacteria in mahi-mahi (Coryphaena hippurus) fillets and dried products. Food Control 2014, 42, 165–171. [Google Scholar] [CrossRef]
- Lu, S.; Ji, H.; Wang, Q.; Li, B.; Li, K.; Xu, C.; Jiang, C. The effects of starter cultures and plant extracts on the biogenic amine accumulation in traditional Chinese smoked horsemeat sausages. Food Control 2015, 50, 869–875. [Google Scholar] [CrossRef]
- Lu, S.; Xu, X.; Shu, R.; Zhou, G.; Meng, Y.; Sun, Y.; Chen, Y.; Wang, P. Characterization of biogenic amines and factors influencing their formation in traditional Chinese sausages. J. Food Sci. 2010, 75, M366–M372. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Mozuriene, E.; Krungleviciute, V.; Novoslavskij, A.; Santini, A.; Rozentale, I.; Juodeikiene, G.; Cizeikiene, D. The impact of lactic acid bacteria with antimicrobial properties on biodegradation of polycyclic aromatic hydrocarbons and biogenic amines in cold smoked pork sausages. Food Control 2017, 71, 285–292. [Google Scholar] [CrossRef]
- Lucas, P.M.W.W. Histamine-Producing Pathway Encoded on an Unstable Plasmid in Lactobacillus hilgardii 0006. Appl. Envirvron. Microb. 2005, 71, 14178. [Google Scholar] [CrossRef] [Green Version]
- Satomi, M.; Mori-Koyanagi, M.; Shozen, K.; Furushita, M.; Oikawa, H.; Yano, Y. Analysis of plasmids encoding the histidine decarboxylase gene in Tetragenococcus muriaticus isolated from Japanese fermented seafoods. Fisheries Sci. 2012, 78, 935–945. [Google Scholar] [CrossRef]
- Landete, J.M.; Pardo, I.; Ferrer, S. Regulation of hdc expression and HDC activity by enological factors in lactic acid bacteria. J. Appl. Microbiol. 2008, 105, 1544–1551. [Google Scholar] [CrossRef]
- Satomi, M.; Furushita, M.; Oikawa, H.; Yoshikawatakahashi, M.; Yano, Y. Analysis of a 30 kbp plasmid encoding histidine decarboxylase gene in Tetragenococcus halophilus isolated from fish sauce. Int. J. Food Microbiol. 2008, 126, 202–209. [Google Scholar] [CrossRef]
- Pereira, C.I.; Crespo, M.T.; Romao, M.V. Evidence for proteolytic activity and biogenic amines production in Lactobacillus curvatus and L. Homohiochii. Int. J. Food Microbiol. 2001, 68, 211–216. [Google Scholar] [CrossRef]
- Tapingkae, W.; Tanasupawat, S.; Parkin, K.L.; Benjakul, S.; Visessanguan, W. Degradation of histamine by extremely halophilic archaea isolated from high salt-fermented fishery products. Enzyme Microb. Tech. 2010, 46, 92–99. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhuang, H.; Chen, X.; Li, L.; Qiao, W.; Zhang, J. Effects of plant polyphenols and α-tocopherol on lipid oxidation, residual nitrites, biogenic amines, and N-nitrosamines formation during ripening and storage of dry-cured bacon. LWT 2015, 60, 199–206. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Van der Meeren, P.; Sampers, I. The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf life of fish and meat products. Food Control 2016, 68, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhang, Y.; Xiao, Z.; Wang, X. Preparation and properties of cinnamon-thyme-ginger composite essential oil nanocapsules. Ind. Crop. Prod. 2018, 122, 85–92. [Google Scholar] [CrossRef]
- Scacchetti, F.A.P.; Pinto, E.; Soares, G.M.B. Functionalization and characterization of cotton with phase change materials and thyme oil encapsulated in beta-cyclodextrins. Prog. Org. Coat. 2017, 107, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Simon-Brown, K.; Solval, K.M.; Chotiko, A.; Alfaro, L.; Reyes, V.; Liu, C.; Dzandu, B.; Kyereh, E.; Goldson Barnaby, A.; Thompson, I.; et al. Microencapsulation of ginger (Zingiber officinale) extract by spray drying technology. LWT 2016, 70, 119–125. [Google Scholar] [CrossRef]
- Fernández, E.; Alegría, Á.; Delgado, S.; Martín, M.C.; Mayo, B. Comparative Phenotypic and Molecular Genetic Profiling of Wild Lactococcus lactis subsp.lactis Strains of the L. lactis subsp. lactis and L. Lactis subsp. cremoris Genotypes, Isolated from Starter-Free Cheeses Made of Raw Milk. Appl. Environ. Microb. 2011, 77, 5324–5335. [Google Scholar] [CrossRef] [Green Version]
- Linares, D.M.; Alvarez-Sieiro, P.; Del Rio, B.; Ladero, V.; Redruello, B.; Martin, M.C.; Fernandez, M.; Alvarez, M.A. Implementation of the agmatine-controlled expression system for inducible gene expression in Lactococcus lactis. Microb. Cell Fact. 2015, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, B.; Linares, D.M.; Ladero, V.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Putrescine production via the agmatine deiminase pathway increases the growth of Lactococcus lactis and causes the alkalinization of the culture medium. Appl. Microbiol. Biot. 2015, 99, 897–905. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Xu, X.; Zhou, G.; Zhu, Z.; Meng, Y.; Sun, Y. Effect of starter cultures on microbial ecosystem and biogenic amines in fermented sausage. Food Control 2010, 21, 444–449. [Google Scholar] [CrossRef]
- Laranjo, M.; Gomes, A.; Agulheiro-Santos, A.C.; Potes, M.E.; Cabrita, M.J.; Garcia, R.; Rocha, J.M.; Roseiro, L.C.; Fernandes, M.J.; Fraqueza, M.J.; et al. Impact of salt reduction on biogenic amines, fatty acids, microbiota, texture and sensory profile in traditional blood dry-cured sausages. Food Chem. 2017, 218, 129–136. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Ladero, V.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Putrescine production by Lactococcus lactis subsp. Cremoris CECT 8666 is reduced by NaCl via a decrease in bacterial growth and the repression of the genes involved in putrescine production. Int. J. Food Microbiol. 2016, 232, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, X.; Chen, H.; Zhang, C.; Kong, B. Impact of spice extracts on the formation of biogenic amines and the physicochemical, microbiological and sensory quality of dry sausage. Food Control 2018, 92, 190–200. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, C.H.; Kwon, E.Y.; Lee, H.J.; Kim, J.Y.; Kim, S.H. Monitoring the contents of biogenic amines in fish and fish products consumed in Korea. Food Control 2010, 21, 1219–1226. [Google Scholar] [CrossRef]
- Dansby, M.A.; Bovell-Benjamin, A.C. Sensory characterization of a ready-to-eat sweetpotato breakfast cereal by descriptive analysis. J. Food Sci. 2003, 68, 706–709. [Google Scholar] [CrossRef]
- Bel Hadj Salah-Fatnassi, K.; Hassayoun, F.; Cheraif, I.; Khan, S.; Jannet, H.B.; Hammami, M.; Aouni, M.; Harzallah-Skhiri, F. Chemical composition, antibacterial and antifungal activities of flowerhead and root essential oils of Santolina chamaecyparissus L., Growing wild in Tunisia. Saudi J. Biol. Sci. 2017, 24, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.; Chuang, T.; Lin, H.; Huang, Y.; Lin, C.; Kung, H.; Tsai, Y. Histamine content and histamine-forming bacteria in dried milkfish (Chanos chanos) products. Food Chem. 2009, 114, 933–938. [Google Scholar] [CrossRef]
- Kimura, B.; Takahashi, H.; Hokimoto, S.; Tanaka, Y.; Fujii, T. Induction of the histidine decarboxylase genes of Photobacterium damselae subsp. Damselae (formally P. histaminum) at low pH. J. Appl. Microbiol. 2009, 107, 485–497. [Google Scholar] [CrossRef]
- Satomi, M.; Furushita, M.; Oikawa, H.; Yano, Y. Diversity of plasmids encoding histidine decarboxylase gene in Tetragenococcus spp. Isolated from Japanese fish sauce. Int. J. Food Microbiol. 2011, 148, 60–65. [Google Scholar] [CrossRef]
- Calles-Enriquez, M.; Eriksen, B.H.; Andersen, P.S.; Rattray, F.P.; Johansen, A.H.; Fernandez, M.; Ladero, V.; Alvarez, M.A. Sequencing and transcriptional analysis of the streptococcus thermophilus histamine biosynthesis gene cluster: Factors that affect differential hdcA expression. Appl. Environ. Microb. 2010, 76, 6231–6238. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Kang, Y.; Hou, W.; Niu, Y.; Kou, X. Microcapsules based on octenyl succinic anhydride (OSA)-modified starch and maltodextrins changing the composition and release property of rose essential oil. Int. J. Biol. Macromol. 2019, 137, 132–138. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Microbial enzymatic activities for improved fermented meats. Trends Food Sci. Tech. 2011, 22, 81–90. [Google Scholar] [CrossRef]
- Coloretti, F.; Chiavari, C.; Poeta, A.; Succi, M.; Tremonte, P.; Grazia, L. Hidden sugars in the mixture: Effects on microbiota and the sensory characteristics of horse meat sausage. LWT 2019, 106, 22–28. [Google Scholar] [CrossRef]
- Ardö, Y. Flavour formation by amino acid catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef]
- Saelao, S.; Maneerat, S.; Thongruck, K.; Watthanasakphuban, N.; Wiriyagulopas, S.; Chobert, J.; Haertlé, T. Reduction of tyramine accumulation in Thai fermented shrimp (kung-som) by nisin Z-producing Lactococcus lactis KTH0-1S as starter culture. Food Control 2018, 90, 249–258. [Google Scholar] [CrossRef]




| Gene | Primer | Sequence (5′→3′) |
|---|---|---|
| hdcA | hdcA-F | GATGGTATTGTTTCKTATGA |
| hdcA-R | CCAAACACCAGCATCTTC | |
| hdcP | hdcP-F | GTCTGATCCATGGACACGGCTGAAC |
| hdcP-R | GTTGCCGCGAATCTAGAATC | |
| hdcB | hdcB-F | TACCGTTAGAGGCGAGTTCC |
| hdcB-R | GGCAGCACAGGATTAGCATC | |
| hisRS | hisRS-F | CACACAGATTGGTTGTGAGGC |
| hisRS-R | CGTCCCGTGTTTCTTTGTCAC | |
| tuf | tuf-F | TCTTCATCATCAACAAGGTCTGCTT |
| tuf-R | GAACACATCTTGCTTTCACGTCAA | |
| recA | recA-F | CAAGGCTTAGAGATTGCCGATG |
| recA-R | ACGAGGAACTAACGCAGCAAC |
| Attribute | Definitions (Developed by the Panel) | |
|---|---|---|
| Appearance | color | The actual hue of the color, pink brown to dark brown |
| gloss | Shiny, attractive marinated color and uniform color | |
| dry | The absence of moistness, resembling dried meat | |
| Flavor | sourness | The smell of oranges |
| odor | Scented with horse meat and marinated flavors | |
| Texture | hardness | Tough and hard to chew |
| organizational structure | Tissue is delicate and elastic |
| Microbiological Counts | Batch | Days (d) | ||||
|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 28 | ||
| LAB | CK | 3.86 ± 0.04 d | 6.68 ± 0.01 c | 7.19 ± 0.01 a | 7.03 ± 0.02 b | 7.11 ± 0.04 ab |
| P | 3.93 ± 0.07 d | 6.81 ± 0.06 c | 7.13 ± 0.01 ab | 7.15 ± 0.02 a | 7.09 ± 0.03 b | |
| PMT | 3.91 ± 0.02 d | 6.59 ± 0.02 c | 7.09 ± 0.02 a | 6.89 ± 0.01 b | 7.01 ± 0.05 a | |
| PO | 3.91 ± 0.03 e | 6.66 ± 0.03 d | 7.13 ± 0.02 b | 6.92 ± 0.04 c | 7.17 ± 0.08 a | |
| GCC+ | CK | 3.97 ± 0.02 c | 6.87 ± 0.01 b | 7.79 ± 0.04 a | 6.63 ± 0.08 b | 6.54 ± 0.02 b |
| P | 3.95 ± 0.01 c | 6.75 ± 0.04 b | 7.46 ± 0.02 a | 6.47 ± 0.03 b | 6.45 ± 0.03 b | |
| PMT | 3.95 ± 0.05 c | 6.59 ± 0.06 b | 7.79 ± 0.02 a | 6.44 ± 0.04 b | 6.26 ± 0.02 b | |
| PO | 3.96 ± 0.03 c | 6.61 ± 0.02 b | 7.56 ± 0.02 a | 6.43 ± 0.04 b | 6.27 ± 0.07 b | |
| Enterobacteria | CK | 3.09 ± 0.07 a | 3.16 ± 0.04 a | 2.92 ± 0.02 b | 2.11 ± 0.04 c | 1.01 ± 0.01 d |
| P | 5.64 ± 0.05 a | 4.92 ± 0.04 b | 4.18 ± 0.06 c | 3.97 ± 0.03 d | 3.36 ± 0.01 e | |
| PMT | 5.47 ± 0.02 a | 4.31 ± 0.01 b | 3.53 ± 0.02 c | 3.09 ± 0.01 d | nd | |
| PO | 5.59 ± 0.07 a | 4.68 ± 0.02 b | 3.96 ± 0.03 c | 3.12 ± 0.01 d | 1.85 ± 0.029 e | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Huang, Y.; Lu, L.; Liu, Y.; Tang, Z.; Lu, S. Impact of Thyme Microcapsules on Histamine Production by Proteus bacillus in Xinjiang Smoked Horsemeat Sausage. Foods 2021, 10, 2491. https://doi.org/10.3390/foods10102491
Yu H, Huang Y, Lu L, Liu Y, Tang Z, Lu S. Impact of Thyme Microcapsules on Histamine Production by Proteus bacillus in Xinjiang Smoked Horsemeat Sausage. Foods. 2021; 10(10):2491. https://doi.org/10.3390/foods10102491
Chicago/Turabian StyleYu, Honghong, Yali Huang, Liliang Lu, Yuhan Liu, Zonggui Tang, and Shiling Lu. 2021. "Impact of Thyme Microcapsules on Histamine Production by Proteus bacillus in Xinjiang Smoked Horsemeat Sausage" Foods 10, no. 10: 2491. https://doi.org/10.3390/foods10102491