Occurrence of Mineral Oil Hydrocarbons in Omega-3 Fatty Acid Dietary Supplements
Abstract
:1. Introduction
2. Materials and Methods
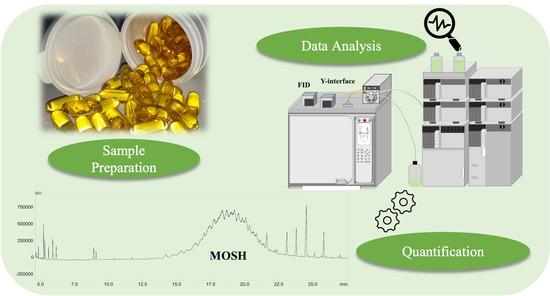
2.1. Samples and Sample Preparation
2.2. Chemicals
2.3. LC-GC Analyses
2.4. Method Validation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority (EFSA). Scientific Opinion on Mineral Oil Hydrocarbons in Food. EFSA J. 2012, 10, 2704. [Google Scholar]
- Grob, K.; Lanfracnhi, M.; Egli, J.; Artho, A. Determination of food contamination by mineral oil from jute sacks using coupled LC-GC. J. Assoc. Off. Anal. Chem. 1991, 74, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Purcaro, G.; Zoccali, M.; Tranchida, P.Q.; Barp, L.; Moret, S.; Conte, L.; Dugo, P.; Mondello, L. Comparison of two different multidimensional liquid–gas chromatography interfaces for determination of mineral oil saturated hydrocarbons in foodstuffs. Anal. Bioanal. Chem. 2013, 405, 1077–1084. [Google Scholar] [CrossRef]
- Moret, S.; Scolaro, M.; Barp, L.; Purcaro, G.; Conte, L.S. Microwave assisted saponification (MAS) followed by on-line liquid chromatography (LC)–gas chromatography (GC) for high-throughput and high-sensitivity determination of mineral oil in different cereal-based foodstuffs. Food Chem. 2016, 196, 50–57. [Google Scholar] [CrossRef]
- Zoccali, M.; Barp, L.; Beccaria, M.; Sciarrone, D.; Purcaro, G.; Mondello, L. Improvement of mineral oil saturated and aromatic hydrocarbons determination in edible oil by liquid–liquid–gas chromatography with dual detection. J. Sep. Sci. 2016, 39, 623–631. [Google Scholar] [CrossRef]
- Canavar, O.; Kappenstein, O.; Luch, A. The analysis of saturated and aromatic mineral oil hydrocarbons in dry foods and from recycled paperboard packages by online HPLC–GC–FID. Food Addit. Contam. A 2018, 35, 2471–2481. [Google Scholar] [CrossRef]
- Van Heyst, A.; Vanlancker, M.; Vercammen, J.; Van den Houwe, K.; Mertens, B.; Elskens, M.; Van Hoeck, E. Analysis of mineral oil in food: Results of a Belgian market survey. Food Addit. Contam. A 2018, 35, 2062–2075. [Google Scholar] [CrossRef]
- Liu, L.; Lia, B.; Ouyangb, J.; Wu, Y. Mild mixed-solvent extraction for determination of total mineral oil hydrocarbon contaminants in milk powder products. Food Chem. 2020, 333, 127488. [Google Scholar] [CrossRef]
- Zoccali, M.; Tranchida, P.Q.; Mondello, L. A lab-developed interface for liquid-gas chromatography coupling based on the use of a modified programmed-temperature-vaporizing injector. J. Chromatogr. A 2020, 1622, 461096. [Google Scholar] [CrossRef] [PubMed]
- Stauff, A.; Schnapka, J.; Heckel, F.; Matissek, R. Mineral oil hydrocarbons (MOSH/MOAH) in edible oils and possible minimization by deodorization through the example of cocoa butter. Eur. J. Lipid Sci. Technol. 2020, 122, 1900383. [Google Scholar] [CrossRef]
- Market Analysis Report. Omega 3 Supplements Market Size, Share & Trends Analysis Report by Source (Fish Oil, Krill Oil), by Form (Soft Gels, Capsules), by Functionality, by End-user, by Distribution Channel, by Region, And Segment Forecasts, 2020–2027. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/omega-3-supplement-market (accessed on 9 September 2021).
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cutuli, D. Functional and structural benefits induced by omega-3 polyunsaturated fatty acids during aging. Curr. Neuropharmacol. 2017, 15, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Thota, R.N.; Ferguson, J.J.A.; Abbott, K.A.; Dias, C.B.; Garg, M.L. Science behind the cardio-metabolic benefits of omega-3 polyunsaturated fatty acids: Biochemical effects vs. clinical outcomes. Food Funct. 2018, 9, 3576–3596. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.; Catena, C.; Novello, M.; Sechi, L.A. Omega-3 Polyunsaturated Fatty Acids in Blood Pressure Control and Essential Hypertension. In Update on Essential Hypertension; Lizbeth, S.-S., Ed.; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Punia, S.; Sandhub, K.S.; Sirohaa, A.K.; Dhull, S.B. Omega 3-metabolism, absorption, bioavailability and health benefits—A review. Pharma Nutr. 2019, 10, 100162. [Google Scholar] [CrossRef]
- Uauy, R.; Gerber, M.; Skeaff, M.; Wolmarans, P. Fats and Fatty Acids in Human Nutrition—Report of an Expert Consultation; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2010. [Google Scholar]
- U.S. Department of Health & Human Services, National Institutes of Health, Office of Dietary Supplements. Available online: https://ods.od.nih.gov/Research/Dietary_Supplement_Label_Database.aspx (accessed on 12 October 2021).
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.K.; Kim, B.; Kim, J.; Lee, K. Analysis of polychlorinated biphenyls (PCBs), heavy metals and omega-3 fatty acids in commercially available Korean functional fish oil supplements. Int. J. Food Sci. Tech. 2016, 51, 2217–2224. [Google Scholar] [CrossRef]
- Olli, J.J.; Breivik, H.; Thorstad, O. Removal of organic environmental pollutants from fish oil using short-path distillation with a working fluid. Chemosphere 2013, 92, 273–278. [Google Scholar] [CrossRef]
- Matsuo, Y.; Nakai, K.; Sakuma, I.; Akutsu, K.; Tatsuta, N.; Ishiyama, M.; Higuchi, T.; Ryuda, N.; Ueno, D. Estimation of Polychlorinated Biphenyls Intake through Fish Oil-Derived Dietary Supplements and Prescription Drugs in the Japanese Population. J. Food Qual. Hazards Control 2019, 6, 146–152. [Google Scholar] [CrossRef]
- Bratinova, S.; Hoekstra, E. Guidance on Sampling, Analysis and Data Reporting for the Monitoring of Mineral Oil Hydrocarbons in Food and Food Contact Materials; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-76-00172-0. [Google Scholar] [CrossRef]
- Zoccali, M.; Salerno, T.M.G.; Tranchida, P.Q.; Mondello, L. Use of a low-cost, lab-made y-interface for liquid-gas chromatography coupling for the analysis of mineral oils in food samples. J. Chromatogr. A 2021, 1648, 462191. [Google Scholar] [CrossRef]
- Sdrigotti, N.; Bauwens, G.; Purcaro, G. A Review of MOSH and MOAH Analysis in Food. LC-GC Europe 2021, 34, 2. [Google Scholar]
- Barp, L.; Kornauth, C.; Wüger, T.; Rudas, M.; Biedermann, M.; Reiner, A.; Concin, N.; Grob, K. Mineral oil in human tissues, Part I: Concentrations and molecular mass distributions. Food Chem. Toxicol. 2014, 72, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Barp, L.; Biedermann, M.; Grob, K.; Blas-Y-Estrada, F.; Nygaard, U.C.; Alexander, J.; Cravedi, J.P. Accumulation of mineral oil saturated hydrocarbons (MOSH) in female Fischer 344 rats: Comparison with human data and consequences for risk assessment. Sci. Total Environ. 2017, 575, 1263–1278. [Google Scholar] [CrossRef] [PubMed]



| Sample N° | Composition (Oil) | Formulation | MOSH (ppm) | CV% (MOSH) | MOAH (ppm) |
|---|---|---|---|---|---|
| 1 | Vegetable + fish | Capsule | 33.0 | 5 | <LoQ |
| 2 | Vegetable + fish | Capsule | 375.7 | 3 | <LoQ |
| 3 | Vegetable + fish | Capsule | 6.3 | 2 | <LoQ |
| 4 | Fish | Capsule | 43.9 | 3 | <LoQ |
| 5 | Fish | Capsule | 92.9 | 4 | <LoQ |
| 6 | Fish | Liquid | 14.4 | 8 | <LoQ |
| 7 | Fish | Capsule | 42.3 | 2 | 9.9 |
| 8 | Fish | Capsule | 2.4 | 8 | <LoQ |
| 9 | Fish | Capsule | 18.4 | 1 | <LoQ |
| 10 | Fish | Capsule | 16.3 | 1 | <LoQ |
| 11 | Fish | Capsule | 4.2 | 3 | <LoQ |
| 12 | Fish + vegetable | Capsule | 65.4 | 4 | <LoQ |
| 13 | Fish | Capsule | 73.5 | <1 | 6.6 |
| 14 | Fish + vegetable | Liquid | 3.1 | 6 | <LoQ |
| 15 | Fish | Capsule | 20.2 | <1 | <LoQ |
| 16 | Microalgae + vegetable | Liquid | 3.8 | 2 | <LoQ |
| 17 | Fatty acid methyl esters | Capsule | 32.8 | 1 | <LoQ |
| MOSH | MOAH | |||
|---|---|---|---|---|
| Sample N° | C-Fraction | ppm | C-Fraction | ppm |
| 1 | C25–C35 C35–C40 C40–C50 | 20.9 10.3 1.8 | - | - |
| 2 | C16–C20 C20–C25 C25–C35 | 60.9 278.8 36.0 | - | - |
| 3 | C20–C25 C25–C35 | 5.3 1.0 | - | - |
| 4 | C25–C35 C35–C40 | 42.5 1.4 | - | - |
| 5 | C20–C25 C25–C35 | 13.3 79.6 | - | - |
| 6 | C25–C35 C35–C40 C40–C50 | 9.1 4.7 0.6 | - | - |
| 7 | C25–C35 C35–C40 | 41.4 0.8 | C25–C35 | 9.9 |
| 8 | C25–C35 | 2.4 | - | - |
| 9 | C25–C35 | 18.4 | - | - |
| 10 | C25–C35 | 16.3 | - | - |
| 11 | C25–C35 C35–C40 | 3.8 0.4 | - | - |
| 12 | C20–C25 C25–C35 C35–C40 | 6.3 55.6 3.5 | - | - |
| 13 | C25–C35 | 73.5 | C25–C35 | 6.6 |
| 14 | C25–C35 C35–C40 | 2.9 0.2 | - | - |
| 15 | C25–C35 C35–C40 | 19.1 1.1 | - | - |
| 16 | C20–C25 C25–C35 | 0.3 3.5 | - | - |
| 17 | C20–C25 C25–C35 C35–C40 | 0.9 31.3 0.6 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arena, A.; Zoccali, M.; Trozzi, A.; Tranchida, P.Q.; Mondello, L. Occurrence of Mineral Oil Hydrocarbons in Omega-3 Fatty Acid Dietary Supplements. Foods 2021, 10, 2424. https://doi.org/10.3390/foods10102424
Arena A, Zoccali M, Trozzi A, Tranchida PQ, Mondello L. Occurrence of Mineral Oil Hydrocarbons in Omega-3 Fatty Acid Dietary Supplements. Foods. 2021; 10(10):2424. https://doi.org/10.3390/foods10102424
Chicago/Turabian StyleArena, Alessia, Mariosimone Zoccali, Alessandra Trozzi, Peter Q. Tranchida, and Luigi Mondello. 2021. "Occurrence of Mineral Oil Hydrocarbons in Omega-3 Fatty Acid Dietary Supplements" Foods 10, no. 10: 2424. https://doi.org/10.3390/foods10102424