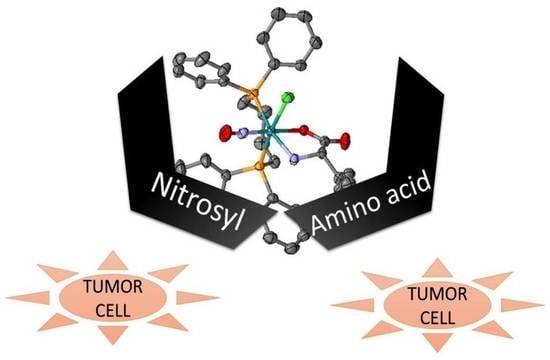
Nitrosyl/Diphenylphosphine/Amino Acid–Ruthenium Complexes as Inhibitors of MDA-MB-231 Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses of the Compounds
2.2. Structural Studies
2.3. 31P{1H}, 13C{1H} and 1H NMR Studies
2.4. Biological Assays
2.4.1. Cytotoxicity of the Ru(II) Complexes
2.4.2. Ru(II) Complexes Induce Apoptosis in Cancer Cells
HSA Fluorescence
DNA Interaction Studies
2.5. Study on the Stability of the Complexes
3. Experimental Section
3.1. Materials for Synthesis
3.2. Instrumentation
3.3. X-ray Crystallography
3.4. Synthesis of Complexes 1–4
3.5. Cell Culture
3.5.1. MTT Assay
3.5.2. Assessment of Apoptosis and Necrosis
3.5.3. Wound Healing Assay
3.6. HSA Fluorescence
3.7. Interaction with DNA
3.7.1. Viscosity
3.7.2. Competitive Trials with Hoechst 33258
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riccardi, C.; Piccolo, M. Metal-Based Complexes in Cancer. Int. J. Mol. Sci. 2023, 24, 7289. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Banerjee, S. Metal-Based Complexes as Potential Anti-cancer Agents. Anti-Cancer Agents Med. Chem. 2022, 22, 2684–2707. [Google Scholar] [CrossRef] [PubMed]
- Gamberi, T.; Hanif, M. Metal-Based Complexes in Cancer Treatment. Biomedicines 2022, 10, 2573. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.; Sjoberg, D.D.; Demac, Q.; Odea, C.; McGill, M.; Tracey, A.; Nogueira, L.; Vickers, A.; Estes, C.; Fine, S.; et al. Phase 2b trial results of padeliporfin (WST11 or Tookad) vascular-targeted photodynamic therapy for partial gland ablation in men with intermediate-risk prostate cancer. J. Clin. Oncol. 2021, 39, e17006. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) Compounds: Next-Generation Anticancer Metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef]
- Coverdale, J.P.C.; Laroiya-McCarron, T.; Romero-Canelón, I. Designing Ruthenium Anticancer Drugs: What Have We Learnt from the Key Drug Candidates? Inorganics 2019, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Murray, B.S.; Dyson, P.J. Recent progress in the development of organometallics for the treatment of cancer. Curr. Opin. Chem. Biol. 2020, 56, 28–34. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, C.Y.; Nam, T.-G. Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Des. Dev. Ther. 2020, 14, 5375–5392. [Google Scholar] [CrossRef]
- Riccardi, C.; Musumeci, D.; Capuozzo, A.; Irace, C.; King, S.; Krauss, I.R.; Paduano, L.; Montesarchio, D. “Dressing up” an Old Drug: An Aminoacyl Lipid for the Functionalization of Ru(III)-Based Anticancer Agents. ACS Biomater. Sci. Eng. 2018, 4, 163–174. [Google Scholar] [CrossRef]
- Leite, C.M.; de Araujo-Neto, J.H.; Corrêa, R.S.; Colina-Vegas, L.; Martínez-Otero, D.; Martins, P.R.; Silva, C.G.; Batista, A.A. On the Cytotoxicity of Chiral Ruthenium Complexes Containing Sulfur Amino Acids against Breast Tumor Cells (MDA-231 and MCF-7). Anti-Cancer Agents Med. Chem. 2021, 21, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Golfeto, C.C.; Von Poelhsitz, G.; Selistre-De-Araújo, H.S.; de Araujo, M.P.; Ellena, J.; Castellano, E.E.; Lopes, L.G.; Moreira, I.S.; Batista, A.A. Synthesis, characterization and cytotoxic activities of the [RuCl2(NO)(dppp)(L)]PF6 complexes. J. Inorg. Biochem. 2010, 104, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, A.P.; da Silva, R.S.; Carneiro, Z.A.; de Carvalho, M.R.; Carvalho, I.; Pernomian, L.; Ferreira, L.P.; Ramos, L.C.; de Souza, G.A.; Formiga, A.L. Improving Cytotoxicity against Breast Cancer Cells by Using Mixed-Ligand Ruthenium(II) Complexes of 2,2′-Bipyridine, Amino Acid, and Nitric Oxide Derivatives as Potential Anticancer Agents. Anti-Cancer Agents Med. Chem. 2021, 21, 1602–1611. [Google Scholar] [CrossRef]
- Mello-Andrade, F.; Guedes, A.P.; Pires, W.C.; Velozo-Sá, V.S.; Delmond, K.A.; Mendes, D.; Molina, M.S.; Matuda, L.; de Sousa, M.A.M.; Melo-Reis, P.; et al. Ru(II)/amino acid complexes inhibit the progression of breast cancer cells through multiple mechanism-induced apoptosis. J. Inorg. Biochem. 2022, 226, 111625. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, T.A.; Von Poelhsitz, G.; Reis, R.I.; Castellano, E.E.; Neves, A.; Lanznaster, M.; Machado, S.P.; Batista, A.A.; Costa-Neto, C.M. A new nitrosyl ruthenium complex: Synthesis, chemical characterization, in vitro and in vivo antitumor activities and probable mechanism of action. Eur. J. Med. Chem. 2011, 46, 3616–3622. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [Green Version]
- Batista, A.A.; Wohnrath, K.; Pereira, C.; Gambardella, M.T.P. Synthesis and characterization of the mer-[RuCl3(NO)(dppb)] isomer. X-ray structures of fac-[RuCl3(NO)(dppm)], cis-[RuCl2(dppm)2] and mer-[RuCl3(NO)(dppb)] [dppm = 1,2-bis(diphenylphosphino)methane and dppb = 1,4-bis(diphenylphosphino)butane]. Polyhedron 1999, 18, 2079–2083. [Google Scholar] [CrossRef]
- Almeida, M.A.P.; Nascimento, F.B.D.; Graminha, A.E.; Ferreira, A.G.; Ellena, J.; Mello, F.M.D.S.; de Lima, A.P.; Silveira-Lacerda, E.D.P.; Batista, A.A. Structural features and cytotoxic activities of [Ru(AA-H)(dppb)(bipy)]PF6 complexes. Polyhedron 2014, 81, 735–742. [Google Scholar] [CrossRef]
- Ghosh, K.; Kumar, S.; Kumar, R. Donation and scavenging of nitric oxide (NO) by flipping of the denticity of carboxylate ligand in novel ruthenium complexes: Photolability of the coordinated NO. Inorg. Chim. Acta 2013, 405, 24–30. [Google Scholar] [CrossRef]
- Schultz, A.J.; Henry, R.L.; Reed, J.; Eisenberg, R. Crystal and molecular structure of trichloronitrosylbis(methyldiphenylphosphine)ruthenium(II), RuCl3(NO)(PMePH2)2. Inorg. Chem. 1974, 13, 732–736. [Google Scholar] [CrossRef]
- Zangl, A.; Klüfers, P.; Schaniel, D.; Woike, T. Photoinduced linkage isomerism of {RuNO}6 complexes with bioligands and related chelators. Dalton Trans. 2009, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Onozuka, T.; Tomizawa, H.; Tanaka, M.; Miki, E. The unexpected reactions of [RuCl3(2mqn)NO]− (H2mqn=2-methyl-8-quinolinol) with 2-chloro-8-quinolinol (H2cqn) and of [RuCl(2cqn)(2mqn)NO] on photoirradiation. Inorg. Chim. Acta 2004, 357, 1303–1308. [Google Scholar] [CrossRef]
- Michael, D.; Mingos, P.; Sherman, D.J. Transition Metal Nitrosyl Complexes. In Advances in Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 1989; Volume 34, pp. 293–377. [Google Scholar] [CrossRef]
- Amaral, M.L.M.D.; Nascimento, R.D.; Silva, L.F.; Arantes, E.C.d.S.; Graminha, A.E.; da Silva, R.S.; Ueno, L.T.; Bogado, A.L.; DeFreitas-Silva, G.; de Lima, R.G. New trans-[Ru(NO)(NO2)(dppb)(o-bdqi)]+ complex as NO donor encapsulated Pluronic F-127 micelles. Polyhedron 2022, 218, 115770. [Google Scholar] [CrossRef]
- Lewandowska, H. Spectroscopic Characterization of Nitrosyl Complexes. In Nitrosyl Complexes in Inorganic Chemistry, Biochemistry and Medicine I; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2013; pp. 115–165. [Google Scholar] [CrossRef]
- De La Cruz, C.; Sheppard, N. A structure-based analysis of the vibrational spectra of nitrosyl ligands in transition-metal coordination complexes and clusters. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 7–28. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Paul, H.; Puranik, V.G.; Lahiri, G.K. Ruthenium mononitro and mononitroso terpyridine complexes incorporating azoimine based ancillary ligands. Synthesis, crystal structure, spectroelectrochemical properties and kinetic aspects. J. Chem. Soc. Dalton Trans. 2001, 481–487. [Google Scholar] [CrossRef]
- Santos, E.R.; Graminha, A.E.; Schultz, M.S.; Correia, I.; Selistre-de-Araújo, H.S.; Corrêa, R.S.; Ellena, J.; Lacerda, E.P.S.; Pessoa, J.C.; Batista, A.A. Cytotoxic activity and structural features of Ru(II)/phosphine/amino acid complexes. J. Inorg. Biochem. 2018, 182, 48–60. [Google Scholar] [CrossRef]
- Rathgeb, A.; Böhm, A.; Novak, M.S.; Gavriluta, A.; Dömötör, O.; Tommasino, J.B.; Enyedy, É.A.; Shova, S.; Meier, S.; Jakupec, M.A.; et al. Ruthenium-Nitrosyl Complexes with Glycine, L-Alanine, L-Valine, L-Proline, D-Proline, L-Serine, L-Threonine, and L-Tyrosine: Synthesis, X-ray Diffraction Structures, Spectroscopic and Electrochemical Properties, and Antiproliferative Activity. Inorg. Chem. 2014, 53, 2718–2729. [Google Scholar] [CrossRef] [Green Version]
- Kalaivani, P.; Saranya, S.; Poornima, P.; Prabhakaran, R.; Dallemer, F.; Padma, V.V.; Natarajan, K. Biological evaluation of new nickel(II) metallates: Synthesis, DNA/protein binding and mitochondrial mediated apoptosis in human lung cancer cells (A549) via ROS hypergeneration and depletion of cellular antioxidant pool. Eur. J. Med. Chem. 2014, 82, 584–599. [Google Scholar] [CrossRef]
- Kratz, F. A clinical update of using albumin as a drug vehicle—A commentary. J. Control. Release 2014, 190, 331–336. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; Volume 4, pp. 853–954. ISBN 978-0-387-46312. [Google Scholar]
- Silva, H.V.R.; Dias, J.S.M.; da Silva, G.F.; Vegas, L.C.; Ionta, M.; Corrêa, C.C.; Batista, A.A.; Barbosa, M.I.F.; Doriguetto, A.C. Phosphine/diimine ruthenium complexes with Cl−, CO, NO+, NO2−, NO3− and pyridine ligands: Pro-apoptotic activity on triple-negative breast cancer cells and DNA/HSA interactions. Polyhedron 2018, 144, 55–65. [Google Scholar] [CrossRef]
- Ramadevi, P.; Singh, R.; Jana, S.S.; Devkar, R.; Chakraborty, D. Ruthenium complexes of ferrocene mannich bases: DNA/BSA interactions and cytotoxicity against A549 cell line. J. Photochem. Photobiol. A Chem. 2015, 305, 1–10. [Google Scholar] [CrossRef]
- Beckford, F.; Thessing, J.; Woods, J.; Didion, J.; Gerasimchuk, N.; Gonzalez-Sarrias, A.; Seeram, N.P. Synthesis and structure of [(η6-p-cymene)Ru(2-anthracen-9-ylmethylene-N-ethylhydrazinecarbothioamide)Cl]Cl; biological evaluation, topoisomerase II inhibition and reaction with DNA and human serum albumin. Metallomics 2011, 3, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacita, N.; Nikolaou, S. Studying the interaction between trinuclear ruthenium complexes and human serum albumin by means of fluorescence quenching. J. Lumin. 2016, 169, 115–120. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Piccolo, M.; Ferraro, M.G.; Graziano, R.; Musumeci, D.; Trifuoggi, M.; Irace, C.; Montesarchio, D. Bioengineered lipophilic Ru(III) complexes as potential anticancer agents. Biomater. Adv. 2022, 139, 213016. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Suárez, J.; Shand, T.; Magliozzo, R.S.; Sánchez-Delgado, R.A. Interactions of arene–Ru(II)–chloroquine complexes of known antimalarial and antitumor activity with human serum albumin (HSA) and transferrin. J. Inorg. Biochem. 2011, 105, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Liu, J.; Li, H.; Zheng, W.; Shi, S.; Chen, L.; Ji, L. Differences in structure, physiological stability, electrochemistry, cytotoxicity, DNA and protein binding properties between two Ru(III) complexes. J. Inorg. Biochem. 2008, 102, 347–358. [Google Scholar] [CrossRef]
- Pages, B.J.; Ang, D.L.; Wright, E.P.; Aldrich-Wright, J.R. Metal complex interactions with DNA. Dalton Trans. 2015, 44, 3505–3526. [Google Scholar] [CrossRef]
- Barra, C.V.; Netto, A.V.G. Antitumour Complexes and DNA Interactions and their Tools of Analysis: An Approach to Metalointercalators. Rev. Virtual Quim. 2015, 7, 1998–2016. [Google Scholar] [CrossRef]
- Wheate, N.J.; Brodie, C.R.; Collins, J.G.; Kemp, S.; Aldrich-Wright, J.R. DNA Intercalators in Cancer Therapy: Organic and Inorganic Drugs and Their Spectroscopic Tools of Analysis. Mini-Rev. Med. Chem. 2007, 7, 627–648. [Google Scholar] [CrossRef]
- Biver, T.; García, B.; Leal, J.M.; Secco, F.; Turriani, E. Left-handed DNA: Intercalation of the cyanine thiazole orange and structural changes. A kinetic and thermodynamic approach. Phys. Chem. Chem. Phys. 2010, 12, 13309–13317. [Google Scholar] [CrossRef]
- Saito, M.; Kobayashi, M.; Iwabuchi, S.-I.; Morita, Y.; Takamura, Y.; Tamiya, E. DNA Condensation Monitoring after Interaction with Hoechst 33258 by Atomic Force Microscopy and Fluorescence Spectroscopy. J. Biochem. 2004, 136, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhou, W.; Yao, X.; Zhao, M.; Li, Y. Determination of nucleic acids based on the fluorescence quenching of Hoechst 33258 at pH 4.5. Anal. Chim. Acta 2006, 570, 21–28. [Google Scholar] [CrossRef]
- Souza, M.L.; Roveda, A.C., Jr.; Pereira, J.C.M.; Franco, D.W. New perspectives on the reactions of metal nitrosyls with thiolates as nucleophiles. Coord. Chem. Rev. 2016, 306, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Roncaroli, F.; Olabe, J.A. The Reactions of Nitrosyl Complexes with Cysteine. Inorg. Chem. 2005, 44, 4719–4727. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. HKL Denzo and Scalepack. In Methods in Enzymology; Carter, C.W., Jr., Sweet, R.M., Eds.; Academic Press: New York, NY, USA, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppens, P.; Leiserowitz, L.; Rabinovich, D. Calculation of absorption corrections for camera and diffractometer data. Acta Crystallogr. 1965, 18, 1035–1038. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bronikowska, J.; Szliszka, E.; Jaworska, D.; Czuba, Z.P.; Krol, W. The Coumarin Psoralidin Enhances Anticancer Effect of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL). Molecules 2012, 17, 6449–6464. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.N.S.A.; Wahab, N.A.; Malek, S.N.A. In Vitro Morphological Assessment of Apoptosis Induced by Antiproliferative Constituents from the Rhizomes of Curcuma zedoaria. Evid.-Based Complement. Altern. Med. 2013, 2013, 257108. [Google Scholar] [CrossRef] [Green Version]
- Rogalska, A.; Marczak, A.; Gajek, A.; Szwed, M.; Śliwińska, A.; Drzewoski, J.; Jóźwiak, Z. Induction of apoptosis in human ovarian cancer cells by new anticancer compounds, epothilone A and B. Toxicol. In Vitro 2013, 27, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Pal, A.; Dey, S.; Chatterjee, B.K.; Chakrabarti, P. Interaction of Virstatin with Human Serum Albumin: Spectroscopic Analysis and Molecular Modeling. PLoS ONE 2012, 7, e37468. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Fragment | (1) | (3) |
|---|---|---|
| Ru(1)−N(2) | 1.742(3) | 1.727(3) |
| Ru(1)−O(1) | 2.024(2) | 2.003(2) |
| Ru(1)−N(1) | 2.150(3) | 2.164(2) |
| Ru(1)−P(1) | 2.4027(10) | 2.3968(8) |
| Ru(1)−P(2) | 2.4004(10) | 2.3975(9) |
| Ru(1)−Cl(1) | 2.4102(10) | 2.4121(9) |
| N(2)−O(3) | 1.141(4) | 1.148(4) |
| N(1)−C(2) | 1.470(4) | 1.489(4) |
| O(1)−C(1) | 1.315(4) | 1.315(4) |
| O(2)−C(1) | 1.213(4) | 1.215(4) |
| C(1)−C(2) | 1.511(5) | 1.521(5) |
| N(2)−Ru(1)−O(1) | 174.23(12) | 176.91(12) |
| N(2)−Ru(1)−N(1) | 94.82(12) | 98.09(12) |
| O(1)−Ru(1)−N(1) | 80.20(10) | 78.82(9) |
| N(2)−Ru(1)−P(1) | 91.64(8) | 91.39(10) |
| O(1)−Ru(1)−P(1) | 81.08(7) | 91.69(6) |
| N(1)−Ru(1)−P(1) | 172.95(7) | 167.31(7) |
| N(2)−Ru(1)−P(2) | 89.16(10) | 96.87(11) |
| O(1)−Ru(1)−P(2) | 95.50(7) | 83.22(7) |
| P(1)−Ru(1)−P(2) | 93.21(4) | 94.97(3) |
| N(2)−Ru(1)−Cl(1) | 89.27(10) | 89.81(11) |
| O(1)−Ru(1)−Cl(1) | 87.37(7) | 89.91(7) |
| N(1)−Ru(1)−Cl(1) | 84.79(8) | 83.10(9) |
| P(1)−Ru(1)−Cl(1) | 89.45(4) | 88.53(4) |
| P(2)−Ru(1)−Cl(1) | 168.34(4) | 172.37(3) |
| O(3)−N(2)−Ru(1) | 172.2(3) | 174.6(3) |
| C(1)−O(1)−Ru(1) | 108.6(2) | 119.1(2) |
| δ 13C ppm (Free Amino Acids) (D2O) | ||||
|---|---|---|---|---|
| Gly | Ala | Phe | Val | |
| C1 | 175.2 (s) | 172.0 (s) | 174.0 (s) | 172.7 (s) |
| C2 | 43.2 (s) | 51.4 (s) | 56.9 (s) | 59.6 (s) |
| C3 | ----- | 33.0 (s) | 37.2 (s) | 30.0 (s) |
| δ 13C ppm (compounds) [RuCl(NO)(AA-H)(dppb)]PF6 (CDCl3) | ||||
| (1) | (2) | (3) | (4) | |
| C1 | 179.4/179.3 (d) (9.7 Hz) | 177.7/177.6 (d) (9.4 Hz) 176.6/176.3 (d) (9.0 Hz) | 179.9/179.8 (d) (9.7 Hz) 178.6/178.5 (d) (8.1 Hz) | 180.8/180.6 (d) (9.6 Hz) 179.8/179.6 (d) (8.1 Hz) |
| C2 | 43.0 (s) | 52.3/50.1 (s) | 56.9/54.0 (s) | 60.6/53.4 (s) |
| C3 | ----- | 37.0/35.0 (s) | 39.0/35.0 (s) | 30.5/29.3.0 (s) |
| Free AA | νs (COO−) (W) | νass (COO−) (S) | Complex | νs (COO−) (W) | νass (COO−) (S) | ν (NO) (S) | Δ(L) | Δ(C) |
|---|---|---|---|---|---|---|---|---|
| Gly | 1413 | 1592 | (1) | 1346 | 1683 | 1880 | 179 | 337 |
| Ala | 1417 | 1595 | (2) | 1379 | 1689 | 1876 | 183 | 310 |
| Phe | 1409 | 1584 | (3) | 1359 | 1690 | 1878 | 173 | 331 |
| Val | 1405 | 1591 | (4) | 1326 | 1686 | 1883 | 177 | 360 |
| Compound | IC50 (μM) | SI | |
|---|---|---|---|
| MDA-MB-231 | L929 | ||
| (1) | 33.6 ± 3.9 | 84.7 ± 3.1 | 2.5 |
| (2) | 25.9 ± 5.8 | 79.3 ± 2.5 | 3.1 |
| (3) | 12.1 ± 0.7 | 37.5 ± 4.7 | 3.1 |
| (4) | 23.9 ± 1.2 | 66.1 ± 4.2 | 2.8 |
| Cisplatin | 12.43 ± 0.20 | 29.0 ± 2.0 | 2.3 |
| Complex | T (K) | Ksv·104 | kq·1012 | r2 | N | Kb | ΔG ° | ΔH ° | ΔS ° |
|---|---|---|---|---|---|---|---|---|---|
| (1) | 298 303 310 | 1.31 ± 0.04 1.40 ± 0.03 1.43 ± 0.03 | 2.62 2.80 2.86 | 0.993 0.996 0.996 | 1.37 1.28 1.22 | 4.81 (±0.29) × 105 1.94 (±0.25) × 105 1.18 (±0.18) × 105 | −32.41 −30.67 −30.10 | −89.85 | −192.72 |
| (2) | 298 303 310 | 1.27 ± 0.04 1.37 ± 0.02 1.36 ± 0.03 | 2.54 2.74 2.67 | 0.991 0.997 0.995 | 1.50 1.48 1.17 | 1.70 (±0.29) × 106 0.75 (±0.28) × 106 0.62 (±0.33) × 106 | −35.55 −34.07 −34.35 | −65.12 | −99.25 |
| (3) | 298 303 310 | 2.28 ± 0.08 2.34 ± 0.04 2.31 ± 0.04 | 4.56 4.68 4.62 | 0.990 0.998 0.997 | 0.89 0.83 0.76 | 8.36 (±0.20) × 103 4.56 (±0.05) × 103 2.50 (±0.06) × 103 | −22.37 −21.22 −20.87 | −77.25 | −184.14 |
| (4) | 298 303 310 | 1.05 ± 0.03 1.14 ± 0.03 1.24 ± 0.03 | 2.10 2.28 2.47 | 0.994 0.994 0.993 | 1.13 1.10 1.04 | 3.62 (±0.13) × 104 3.16 (±0.13) × 104 1.90 (±0.14) × 104 | −26.00 −26.10 −25.40 | −41.05 | −50.48 |
| (1) | (3) | |
|---|---|---|
| Empirical formula | C30H32ClF6N2O3P3Ru·0.5 H2O | C37H38ClF6N2O3P3Ru·CH3OH |
| Molecular weight | 821.01 | 934.17 |
| Temperature (K) | 293(2) K | 293(2) K |
| Wavelength (MoKα) (Å) | 0.71073 Å | 0.71073 Å |
| Crystal system | Monoclinic | Orthorhombic |
| Space group | P21/n | P212121 |
| Unit cell dimensions (Å; °) | a = 11.0010(4) Å b = 10.8570(5) Å; β = 98.649(4)°. c = 28.8450(15) Å | a = 9.26250(10) Å b = 14.6732(3) Å c = 30.1005(6) Å |
| Volume | 3406.0(3) Å3 | 4090.97(12) Å3 |
| Z | 4 | 4 |
| Density (calculated; Mg/m3) | 1.601 Mg/m3 | 1.517 Mg/m3 |
| Absorption coefficient (mm−1) | 0.749 mm−1 | 0.635 mm−1 |
| F(000) | 1660 | 1904 |
| Crystal size (mm3) | 0.06 × 0.14 × 0.22 mm3 | 0.11 × 0.18 × 0.31 mm3 |
| Theta range for data collection (°) | 3.05 to 26.73°. | 2.93 to 26.74°. |
| Index ranges | −11 ≤ h ≤ 13, −12 ≤ k ≤ 13, −35 ≤ l ≤ 36 | −8 ≤ h ≤ 11, −18 ≤ k ≤ 18, −38 ≤ l ≤ 38 |
| Reflections collected | 17,276 | 24,378 |
| Independent reflections | 7182 [R(int) = 0.0571] | 8659 [R(int) = 0.0280] |
| GoF | 1.072 | 1.095 |
| Max. and min. transmission | 0.948 and 0.876 | 0.936 and 0.852 |
| R1; wR2 [I > 2σ (I)] | 0.0453; 0.0963 | 0.0395; 0.0876 |
| R1; wR2 (Total) | 0.0841; 0.1063 | 0.0416; 0.0889 |
| Largest diff. peak and hole | 0.478 and −0.625 e·Å−3 | 0.500 and −0.352 e·Å−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, M.I.F.; Corrêa, R.S.; Guedes, A.P.M.; Graça, A.M.; Andrade, F.M.; Leite, C.M.; Silveira-Lacerda, E.P.; Ellena, J.; Reis, H.V.; Doriguetto, A.C.; et al. Nitrosyl/Diphenylphosphine/Amino Acid–Ruthenium Complexes as Inhibitors of MDA-MB-231 Breast Cancer Cells. Inorganics 2023, 11, 270. https://doi.org/10.3390/inorganics11070270
Barbosa MIF, Corrêa RS, Guedes APM, Graça AM, Andrade FM, Leite CM, Silveira-Lacerda EP, Ellena J, Reis HV, Doriguetto AC, et al. Nitrosyl/Diphenylphosphine/Amino Acid–Ruthenium Complexes as Inhibitors of MDA-MB-231 Breast Cancer Cells. Inorganics. 2023; 11(7):270. https://doi.org/10.3390/inorganics11070270
Chicago/Turabian StyleBarbosa, Marília I. F., Rodrigo S. Corrêa, Adriana P. M. Guedes, Alex M. Graça, Francyelli M. Andrade, Celisnólia M. Leite, Elisângela P. Silveira-Lacerda, Javier Ellena, Henrique V. Reis, Antônio C. Doriguetto, and et al. 2023. "Nitrosyl/Diphenylphosphine/Amino Acid–Ruthenium Complexes as Inhibitors of MDA-MB-231 Breast Cancer Cells" Inorganics 11, no. 7: 270. https://doi.org/10.3390/inorganics11070270