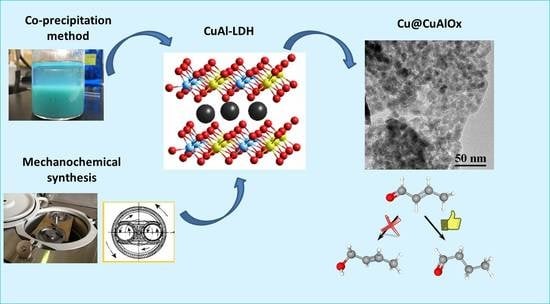
Synthesis of CuAl-LDHs by Co-Precipitation and Mechanochemical Methods and Selective Hydrogenation Catalysts Based on Them
Abstract
:1. Introduction
2. Results
2.1. Synthesis of CuAl-LDH by the Co-Precipitation Method
2.2. Synthesis of CuAl-LDH by Mechanochemical Method
2.3. Investigation of the Characteristics of the Synthesized LDHs
2.4. The Formation of Catalysts Based on CuAl-LDH
2.5. Catalytic Properties in the Liquid-Phase Hydrogenation of Crotonaldehyde
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belskaya, O.B.; Likholobov, V.A. Mechanochemical Synthesis of Layered Double Hydroxides as a Promising Method for the Preparation of Adsorbents and Catalysts. Kinet. Catal. 2022, 63, 695–723. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Leont’eva, N.N.; Gulyaeva, T.I.; Cherepanova, S.V.; Talzi, V.P.; Drozdov, V.A.; Likholobov, V.A. Influence of a Doubly Charged Cation Nature on the Formation and Properties of Mixed Oxides MAlOx (M = Mg2+, Zn2+, Ni2+) Obtained from the Layered Hydroxide Precursors. Russ. Chem. Bul. 2013, 62, 2349–2361. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Mironenko, R.M.; Gulyaeva, T.I.; Trenikhin, M.V.; Muromtsev, I.V.; Trubina, S.V.; Zvereva, V.V.; Likholobov, V.A. Catalysts derived from nickel-containing layered double hydroxides for aqueous-phase furfural hydrogenation. Catalysts 2022, 12, 598. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Stepanova, L.N.; Gulyaeva, T.I.; Leont’eva, N.N.; Zaikovskii, V.I.; Salanov, A.N.; Likholobov, V.A. Synthesis of Mg2+-, Al3+-, and Ga3+-Containing Layered Hydroxides and Supported Platinum Catalysts Based Thereon. Kinet. Catal. 2016, 57, 546–556. [Google Scholar] [CrossRef]
- Sato, K.; Abe, N.; Kawagoe, T.; Miyahara Sh Honda, K. Supported Ni catalysts prepared from hydrotalcite-like compounds for the production of hydrogen by ammonia decomposition. Int. J. Hydrog. Energy 2017, 42, 6610–6617. [Google Scholar] [CrossRef]
- Su, Q.; Wang, H.; Gu, L.; Ji, W.; Au, C.T. Fe-based catalyst derived from MgFe-LDH: Very efficient yet simply obtainable for hydrogen production via ammonia decomposition. Int. J. Hydrog. Energy 2021, 46, 31122–31132. [Google Scholar] [CrossRef]
- Rosset, M.; Féris, L.; Perez-Lopez, O.W. Biogas dry reforming using Ni–Al-LDH catalysts reconstructed with Mg and Zn. Int. J. Hydrog. Energy 2021, 46, 20359–20376. [Google Scholar] [CrossRef]
- Starukh, G. Photocatalytically enhanced cationic dye removal with Zn-Al layered double hydroxides. Nanoscale Res. Lett. 2017, 12, 391–398. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Li, X.; Bao, W.; Jin Ch Feng, X.; Liu, G.; Yang, C.; Zhang, N. Chromium-Modified Ultrathin CoFe-LDH as High-Efficiency Electrode for Hydrogen Evolution Reaction. Nanomaterials 2022, 12, 1227. [Google Scholar] [CrossRef]
- Bao, W.; Yang, C.; Ai, T.; Zhang, J.; Zhou, L.; Li, Y.; Wei, X.; Zou, X.; Wang, Y. Modulating interfacial charge distribution of NiSe nanoarrays with NiFe-LDH nanosheets for boosting oxygen evolution reaction. Fuel 2023, 332, 126227. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V.; Bulavchenko, O.A.; Bukhtiyarov, A.V.; Nuzhdin, A.L.; Bukhtiyarova, G.A. Selective Hydrogenation of 5-Acetoxymethylfurfural over Cu-Based Catalysts in a Flow Reactor: Effect of Cu-Al Layered Double Hydroxides Synthesis Conditions on Catalytic Properties. Catalysts 2022, 12, 878. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Likholobov, V.A. Aqueous-phase hydrogenation of furfural in the presence of supported metallic catalysts of different types. Review. Dokl. Chem. 2023, 509. in press. [Google Scholar]
- Bukhtiyarova, M.V.; Nuzhdin, A.L.; Kardash, T.Y.; Bukhtiyarov, A.V.; Gerasimov, E.Y.; Romanenko, A.V. n-Methylation of p-anisidine over the catalysts based on Cu-containing layered double hydroxides. Kinet. Catal. 2019, 60, 343–354. [Google Scholar] [CrossRef]
- Gao, D.; Han, F.; Waterhouse, G.I.N.; Li, Y.; Zhang, L. A highly efficient iron phthalocyanine-intercalated CuFe-LDH catalyst for the selective oxidation of 5-hydroxymethylfurfural to 5-formyl-2-furanic acid. Catal. Commun. 2023, 173, 106561. [Google Scholar] [CrossRef]
- Zheng, H.; Narkhede, N.; Zhang, G.; Zhang, H.; Ma, L.; Yu, S. Highly dispersed Cu catalyst based on the layer confinement effect of Cu/Zn/Ga-LDH for methanol synthesis. Mol. Catal. 2021, 516, 111984. [Google Scholar] [CrossRef]
- Yusuf, S.; Dinari, M.; Ohe, A. Facial synthesis of V-containing CuMgAl-LDHs as a new catalyst for the phenol hydroxylation. Chem. Phys. 2021, 546, 111183. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Jing, M.; Tang, S.; Wu, Y.; Liu, W. Synthesis of CuNiSn LDHs as highly efficient Fenton catalysts for degradation of phenol. Appl. Clay Sci. 2020, 186, 105433. [Google Scholar] [CrossRef]
- Song, J.; Wang Sh Xu, Y.; Liu, Q.; Zhao, Y. LDH derived MgAl2O4 spinel supported Pd catalyst for the low-temperature methane combustion: Roles of interaction between spinel and PdO. Appl. Cat. A Gen. 2021, 621, 118211. [Google Scholar] [CrossRef]
- Yamaoka, T.; Abe, M.; Tsuji, M. Synthesis of Cu-Al hydrotalcite like compound and its ion exchange property. Mater. Res. Bull. 1989, 24, 1183–119938. [Google Scholar] [CrossRef]
- Alejandre, A.; Medina, F.; Salagre, P.; Correig, X.; Sueiras, J.E. Preparation and study of Cu-Al mixed oxides via hydrotalcitelike precursors. Chem. Mater. 1999, 11, 939–948. [Google Scholar] [CrossRef]
- Lwin, Y.; Yarmo, M.A.; Yaakob, Z.; Mohamad, A.B.; Daud, W.R.W. Synthesis and characterization of Cu-Al layered double hydroxides. Mater. Res. Bull. 2001, 36, 193–198. [Google Scholar] [CrossRef]
- Trujillano, R.; Holgado, M.J.; Pigazo, F.; Rives, V. Preparation, physicochemical characterisation and magnetic properties of Cu–Al layered double hydroxides with CO32− and anionic surfactants with different alkyl chains in the interlayer. Phys. B 2006, 373, 267–273. [Google Scholar] [CrossRef]
- Britto, S.; Kamath, P.V. Thermal, solution and reductive decomposition of Cu-Al layered double hydroxides into oxide products. J. Solid. State Chem. 2009, 182, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid. State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Chen, Y.; Liu, T.; Liu, C.; Zhang, X.; Yi, M.; Chu, Z.; Han, X. A Novel three-dimensional hierarchical CuAl layered double hydroxide with excellent catalytic activity for degradation of methyl orange. RSC Adv. 2017, 7, 29051. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; He, X.; Chen, M.; Hu, H.; Zhang, Q.; Liu, X. Mechanochemical synthesis of Cu-Al and methyl orange intercalated Cu-Al layered double hydroxides. Mater. Chem. Phys. 2017, 191, 173–180. [Google Scholar] [CrossRef]
- Qu, J.; He, X.; Lei, Z.; Zhang, Q.; Liu, X. Mechanochemical synthesis of dodecyl sulfate anion (DS−) intercalated Cu-Al layered double hydroxide. Solid. State Sci. 2017, 74, 125–130. [Google Scholar] [CrossRef]
- Barnard, B.A.; Labuschagné, F.J.W.J. Exploring the Wet Mechanochemical Synthesis of Mg-Al, Ca-Al, Zn-Al and Cu-Al Layered Double Hydroxides from Oxides, Hydroxides and Basic Carbonates. Crystals 2020, 10, 954. [Google Scholar] [CrossRef]
- Bai, J.; Xu, J.; Ma, M.; Liu, H.; Cai, M.; Cheng, Q.; Wei, Y.; Guo, L.; Chen, F.; Chen, J.; et al. Efficient Nisingle bondIr alloy catalyst for selective hydrogenation of benzonitrile, crotonaldehyde and benzylideneacetone. Catal. Commun. 2023, 176, 106630. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Chen, L.; Li, Z.; Liu, W.; Xu, E.; Zhang, Y.; Hong, S.; Zhang, X.; Wei, M. NiBi intermetallic compounds catalyst toward selective hydrogenation of unsaturated aldehydes. Appl. Catal. B. Environ. 2020, 277, 119273. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Belskaya, O.B.; Vasilevich, A.V.; Leont’eva, N.N.; Baklanova, O.N.; Likholobov, V.A. Effect of the Composition of Initial Components and the Conditions of Activation on the Mechanochemical Synthesis of Magnesium–Aluminum Layered Double Hydroxides. Kinet. Catal. 2018, 59, 521–531. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Avilés, Y.; Borau, V.; Luque, J.M.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Thermal decomposition of Mg/Al and Mg/Ga layered-double hydroxides: A spectroscopic study. J. Mater. Chem. 1999, 9, 1603–1607. [Google Scholar] [CrossRef]
- Segal, S.R.; Carrado, K.A.; Marshall, C.L.; Anderson, K.B. Catalytic decomposition of alcohols, including ethanol, for in situ H2 generation in a fuel stream using a layered double hydroxide-derived catalyst. Appl. Catal. A Gen. 2003, 248, 33–45. [Google Scholar] [CrossRef]
- Bridier, B.; López, N.; Pérez-Ramírez, J. Partial hydrogenation of propyne over copper-based catalysts and comparison with nickel-based analogues. J. Catal. 2010, 269, 80–92. [Google Scholar] [CrossRef]
- Dewangan, N.; Hui, W.M.; Jayaprakash, S.; Bawah, A.R.; Poerjoto, A.J.; Jie, T.; Jangam, A.; Hidajat, K.; Kawi, S. Recent progress on layered double hydroxide (LDH) derived metal-based catalysts for CO2 conversion to valuable chemicals. Catal. Today 2020, 356, 490–513. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Raj, K.J.A.; Prakash, M.G.; Mahalakshmy, R.; Elangovan, T.; Viswanathan, B. Liquid phase hydrogenation of crotanaldehyde over nickel supported on titania. J. Molec Catal. A Chem. 2013, 366, 92–98. [Google Scholar]












| Sample | SBET, m2 g−1 | Vads, cm3 g−1 | CSR (111), Å |
|---|---|---|---|
| CuAl-ma-300 | 77 | 0.26 | 173 |
| CuAl-ma-550 | 53 | 0.21 | 308 |
| CuAl-cp-300 | 99 | 0.37 | 70 |
| CuAl-cp-550 | 71 | 0.30 | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belskaya, O.B.; Terekhova, E.N.; Gorbunova, O.V.; Muromtsev, I.V.; Trenikhin, M.V.; Salanov, A.N.; Likholobov, V.A. Synthesis of CuAl-LDHs by Co-Precipitation and Mechanochemical Methods and Selective Hydrogenation Catalysts Based on Them. Inorganics 2023, 11, 247. https://doi.org/10.3390/inorganics11060247
Belskaya OB, Terekhova EN, Gorbunova OV, Muromtsev IV, Trenikhin MV, Salanov AN, Likholobov VA. Synthesis of CuAl-LDHs by Co-Precipitation and Mechanochemical Methods and Selective Hydrogenation Catalysts Based on Them. Inorganics. 2023; 11(6):247. https://doi.org/10.3390/inorganics11060247
Chicago/Turabian StyleBelskaya, Olga B., Elena N. Terekhova, Oksana V. Gorbunova, Ivan V. Muromtsev, Mikhail V. Trenikhin, Aleksei N. Salanov, and Vladimir A. Likholobov. 2023. "Synthesis of CuAl-LDHs by Co-Precipitation and Mechanochemical Methods and Selective Hydrogenation Catalysts Based on Them" Inorganics 11, no. 6: 247. https://doi.org/10.3390/inorganics11060247