1. Introduction
A lot of research has been performed on
N-heterocyclic carbenes (NHCs) since the isolation of the first free carbene by Arduengo et al. in 1991 [
1]. Such molecules are exceptional δ−donating ligands due to the lone pair of electrons, which is either sp (triplet carbene) or sp
2 (singlet carbene) hybridized. They can be compared to phosphines in terms of binding, albeit stronger [
2]. NHC ligands can also be compared with each other [
3,
4]. In terms of the catalysis, the most famous NHC Pd system would be the so-called PEPPSI system (PEPPSI: pyridine-enhanced precatalyst preparation stabilization and initiation;
Scheme 1). The air- and moisture-stable complexes are also known for their straightforward synthetic approach [
5,
6,
7,
8,
9].
While NHC metal complexes are usually very stable, even under harsh conditions [
10,
11,
12,
13,
14,
15,
16,
17], their role in catalysis-related applications is strongly debated [
6,
15,
18,
19,
20,
21,
22,
23,
24]. In the case of palladium-based systems, both heterogenous as well as homogenous catalysis mechanisms have been observed. It has been proposed that the formally stable carbene complex decomposes under the reaction conditions to release Pd nanoparticles, which are stabilized by a shell of positively charged azolium ions (i.e., the liberated NHC precursor). These Pd clusters are believed to represent the actual catalyst [
22]. Usually, the NHC precursors are based on imidazole and its derivatives (e.g., benzimidazole) [
15,
25,
26,
27,
28,
29,
30,
31]. One example of a triazolium precursor, which is much less widely used in general, is also known [
30]. Unlike for other NHC precursors, the use of naturally occurring xanthine derivatives avoids multiple-step synthesis. Thus, this strategy basically fulfills the criteria of green chemistry, as defined by Anastas and Warner [
32]. Moreover, the NHC moiety is non-toxic and widely abundant [
5]; the most prominent representatives, i.e., caffeine and theophylline, can readily be modified by simple chemical transformations, thus enabling the modular synthesis of bio-based NHC precursors [
33]. The purine alkaloids from the xanthine family can be extracted from natural resources using supercritical CO
2 as the solvent, followed by purification via ion-exchange chromatography with water, as the eluent. Various protocols for the environmentally friendly process are available [
34,
35,
36,
37].
It can be expected that the concept of using complexes of bio-based NHC ligands in catalysis bears an enormous potential, which is largely unexplored and, to date, is still very limited in scope. However, the enormous interest in xanthine-based complexes as organometallic catalysts is reflected by the vastly increasing number of publications in this field [
8,
17,
19,
23,
29,
33,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58]. Caffeine-based NHC-Pd(II) complexes have already been studied regarding their catalytic properties; however, the mechanism of catalysis has only been elucidated in a single case [
5,
8,
19,
23,
29,
55,
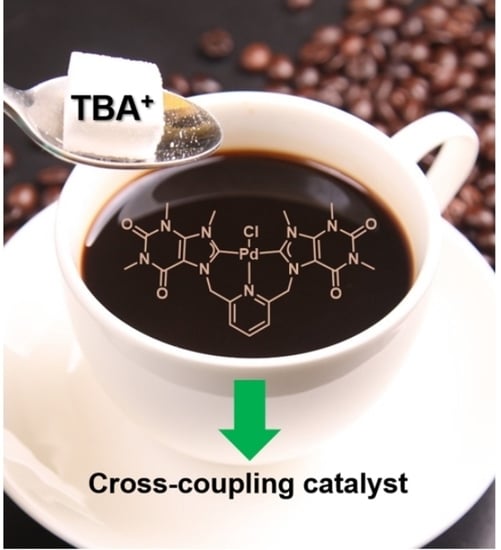
59]. We recently reported the synthesis and structural characterization of a Pt(II) and a Pd(II) complex bearing a tridentate
bis-NHC caffeine-based ligand. palladium complex [
57]. Following up on this, the latter, complex
1 (
Scheme 2) is now studied regarding its catalytic activity in various cross-coupling reactions.
2. Results
We started our catalysis studies with the Suzuki cross-coupling reaction between aryl halides and phenyl boronic acid. For this purpose,
p-nitro-bromobenzene, a commonly used model substrate, was selected to elaborate the optimal reaction conditions. The catalyst loading was kept constant at 1 mol%. The results are summarized in
Table 1.
The optimal conditions were determined to be a mixture of THF and water in a 2:1 ratio with K
2CO
3 as the base (Entry 4). The yield of the reactions in water was very low. This is most likely due to the insolubility of the reactant. As expected, a higher reaction temperature also increased the yield (Entries 4, 7 and 8) and, thus, all further reactions were performed at 70 °C. The effect of a surfactant, e.g., tetrabutylammonium bromide (TBABr), was also tested (Entries 9 and 10). It has been proposed that the azolium salt forms a protective, yet permeable shell around the Pd particles, thus increasing the catalyst’s efficiency. A decrease in yield was observed in the absence of TBABr (19 and 65% vs. 42 and 80% at 40 and 50 °C, respectively). No Pd black was observed when TBABr was present; whereas black particles formed if it was absent. Due to its beneficial role, this additive was thus always used in further reactions. Next, we tested the catalyst under the optimized reaction conditions for different substrates (
Figures S1–S3). We chose chloro-, bromo- and iodo-substituted aryl compounds with electron-withdrawing, electron-donating or neutral substituents. The results are listed in
Table 2.
Overall, the cross-coupling reaction proceeded quite quickly with electron-poor or iodo-substituted aryl substrates. On the other hand, electron-donating substituents, such as the methoxy group, showed the worst performance.
The Mizoroki–Heck cross-coupling reaction, catalyzed by the Pd(II) complex
1, was studied next. Similar to the aforementioned Suzuki reaction,
p-nitro-bromobenzene was used as the model substrate in combination with methyl acrylate to identify the optimal reaction conditions. The results of the screening experiments are summarized in
Table 3.
In summary, the highest yield was obtained with NMP and K
2CO
3, as the solvent and base, respectively (Entry 4). However, in an aqueous solution, even if the surfactant TBABr was present, substrate conversion did not occur. Based on these optimized conditions, different substrates (vide supra) were tested in order to identify any substituent effects (
Table 4,
Figures S4–S6).
Unlike for the Suzuki coupling, electron-poor aryl chlorides can be coupled with methyl acrylate (entry 3). For the aryl iodides (entries 7–9) as well as electron-poor aryl bromides (Entry 6), the reaction was very fast and full substrate conversion was reached within two hours. The methoxy-substituted and unsubstituted aryl bromides (entries 4 and 5) revealed the worst performance with the yields being lower when compared to those of the corresponding Suzuki reactions.
Finally, the applicability of
1 to act as catalyst in the
Sonogashira cross-coupling between phenylacetylene and
p-nitro-bromobenzene was investigated. The results of the reaction-optimization studies are shown in
Table 5.
The optimal reaction conditions for the
Sonogashira cross-coupling were found to be DMAc, as the solvent, piperidine, as the base, and a reaction temperature of 100 °C. While the reaction was even faster at 120 °C, it did not reach completion and some decomposition was observed. As in the previous cases, the presence of TBABr again improved the overall performance of the reaction. The formation of Pd black was not observed when the surfactant was added. The same substrates as for the other two cross-coupling reactions were employed under the optimized conditions (
Figures S7–S9). The results are summarized in
Table 6.
Surprisingly, both chlorobenzene and 4-nitro-chlorobenzene reacted under these conditions. However, the reaction plateaued off very quickly when compared to the others: after a reaction time of 4 h, no further substrate conversion when compared to the reaction rate after 2 h could be observed. This finding might be due to the copper salt which might only be available for a limited number of turnovers (TONs). Regardless, the general trend that electron-donating substituents lowered the substrate reactivity retained; the catalyst was not capable to counteract this (Entries 2 and 5). Only the aryl iodides exhibited a very high reactivity.
We also wanted to check how active the catalyst was, and, what its performance at very low loading would be. The Mizoroki–Heck reaction was chosen and iodobenzene was coupled with methyl acrylate at a catalyst loading of 1 ppm (
Figures S10–S14). This resulted in a conversion of 98% after 30 h with a turnover number of 1.44 × 10
6 and a turnover rate of 48,145/h.
3. Discussion
Whether the catalysis stems from the palladium complex or a ligand-less species is not obvious and must be determined via control experiments. One indication of the reaction mechanism is the kinetics of the reaction. If the catalyst is homogeneous, then a linear kinetics will be observed. A visible induction period, however, points towards an active catalyst, which must be generated, and, thus, the kinetics possesses a sigmoidal shape [
60]. For the Mizoroki–Heck reaction with the activated aryl chloride (
Table 4, Entry 3) this was observed. After two hours, only 24% yield was observed, whereas the reaction was almost completed after four hours. Another such experiment is the catalyst poisoning test which will deactivate monomeric as well as Pd clusters, which could be the active species. We tested for catalyst poisoning by adding a large excess of poly(4-vinylpyridine) (PVP) to the reaction vessel prior to heating (
Table 1, Entry 11). This is commonly known as the “mercury test”, and PVP performs the same role as poison, so mercury does not have to be used. Similar to mercury, this polymer strongly interacts with Pd nanoparticles, which are formed during the reaction, and renders them incapable of catalysis [
22]. As the reaction did not proceed at all under these conditions, we conclude that the catalytic system is heterogeneous: Compound
1 releases colloidal Pd(0), and thus represents only the precursor to the actual catalyst. Notably, this particular method of testing was further investigated by Ananikov et al. [
61]. These authors reported a dependence on the poison loading and that the mechanisms and false positive results need to be considered. For these reasons, the so-called “mercury-poisoning test” was identified as a mostly qualitative tool. Regardless, the absence of catalysis does point towards a heterogeneous system. While these considerations are new in relation to NHC-based palladium complexes, for the Mizoroki–Heck coupling reaction, this type of mechanism is known. In particular, pincer complexes were shown to be precursors for monomeric Pd particles, which in turn were the active catalyst [
62,
63,
64,
65]. With all of this evidence, the mechanism of the catalysis of complex
1 is most likely of a heterogeneous nature.
The low performance of the reactions in water is most likely due to the low solubility in this solvent and the addition of alcohol was deemed an effective strategy by other authors [
5]. The overall low catalyst performance regarding the conversion of aryl chlorides can be rationalized by the low rate of oxidative addition which is well-known for chlorides compared to bromides and iodides [
66]. The strong dependence of the activity of
1 on the presence of additives might be rationalized by the highly polar caffeine moiety and its strong dipole moment [
67]. This allows for the caffeine to be included in the ionic shell which stabilizes the Pd particles.
The obtained results can be compared to those reported recently by other researchers (
Scheme 3).
In one example, Rahman et al. [
8] tested different xanthine–palladium complexes (
2, 3) with respect to their catalytic activity for the Heck and Sonogashira cross-coupling reaction; however, this study was limited to electron-withdrawing phenylacetylenes. Complex
1, presented herein, allows a significantly faster substrate conversion (i.e., 2 h vs. 15 h) and a lower catalyst loading (i.e., 1 mol% vs. 2 mol%), at least when comparing electron-withdrawing coupling partners. As one striking difference between Rahman’s work and our studies, the utilization of additives, such as TBABr, is worth mentioning. As we have shown, such additives have a beneficial effect on the reaction and can lead to shorter reaction times.
The results of the Suzuki reaction can also be discussed in the context of Delaude’s recent studies [
5]. The authors employed the caffeine-derived PEPPSI-type NHC-palladium complexes
4 and
5 as the catalyst for the Suzuki reaction of a large array of substrates and under varying conditions [
9,
27]. The PEPPSI concept relies on the presence of a labile pyridine ligand, whose displacement under the reaction conditions generates the catalytically active species, and thus opens the catalytic cycle [
7]. It was shown that such complexes provided good yields and high degrees of substrate conversion (>80%) under very mild reaction conditions (40 °C) and in short reaction times (2 h) [
5]. The addition of alcohol to water enabled them to perform the cross-coupling in aqueous media, as the improved solubility enhanced the reaction. A chloride ligand at the palladium led to better performance compared to an iodide ligand. However, these authors also did not pay attention to the influence of additives (e.g., the surfactant TBABr).
Finally, we also want to compare the performance of compound
1 with that of a similar catalyst,
6, which was used by Nielsen et al.; this complex contains
N-methyl imidazole moieties instead of the caffeine ones [
68]. The Heck reaction with electron-withdrawing aryl bromides and chlorides was studied. In the case of the bromide, quantitative substrate conversion within 21 h at a catalyst loading of 1 mol% was reported, whereas the chloride afforded a yield of only 11% after 100 h at the same catalyst loading. In both cases, the reaction temperature was set o 120 °C. Remarkably, the authors investigated the influence of an additive (i.e., a tertiary ammonium salt) and observed a reduction in the catalyst’s activity (i.e., in the presence of the additive, the conversion decreased to 35%). In comparison, the herein presented complex
1 exhibited a superior activity towards the conversion of aryl chlorides, as expressed by the shorter reaction times as well as the capability to even couple electron-withdrawing derivatives.
We also want to mention here that the amount of Pd used in the reaction can be reduced to as low as 1 ppm, while the reaction rate still remains at acceptable levels. This is especially important when economical as well as ecological aspects are considered.
4. Materials and Methods
All reagents and analytical-grade solvents were purchased from commercial suppliers and used as received. Poly(4-vinylpyridine) (PVP,
MW ≈ 60,000 g mol
−1) was purchased from Sigma-Aldrich. Complex
1 was synthesized according to the previous published procedure [
57]. The chromatographic purification of the cross-coupling products was performed using silica gel 60 (Merck). In all cases, the reaction mixtures were purged with N
2 for 20 min before starting the reactions.
1H NMR spectra were recorded at 25 °C on Bruker AVANCE instruments (250, 300 or 400 MHz) in deuterated solvents (Euriso-Top). Chemical shifts are given in ppm and referenced to the solvent signal. The spectroscopic data of the products match the literature.
Experimental Section
General procedure for the Suzuki coupling
The respective aryl halide (0.1 mmol, 1.0 eq.), base (4.0 eq.), tetrabutylammonium bromide (1.0 eq.), 1 (1 mol%) and phenylboronic acid (2.5 eq.) were placed in an oven-dried and N2-purged flask. The respective solvents (2 mL) were added via a septum and the reaction mixture was stirred at the respective temperature for up to 24 h. Subsequently, the mixture was extracted with chloroform (20 mL) and washed with water (50 mL). The solvent was evaporated under reduced pressure, and the residue was subjected to a column-chromatographic separation (silica gel 60, hexane/ethyl acetate, 2:1 as eluent).
General procedure for the Sonogashira coupling
The aryl halide (0.1 mmol, 1.0 eq.), base (4.0 eq.), tetrabutylammonium bromide (1.0 eq.), 1 (1 mol%), CuI (1 mol%) and phenylacetylene (3.0 eq.) were placed in an oven-dried and N2-purged flask. The solvents (2 mL) were added via a septum and the reaction mixture was stirred at the given temperature for up to 24 h. Subsequently, the mixture was extracted with chloroform (20 mL) and washed with water (50 mL). The solvent was evaporated under reduced pressure and the residue was subjected to a column-chromatographic separation (silica gel 60, hexane/ethyl acetate, 2:1 as eluent).
General procedure for the Mizoroki–Heck coupling
The respective aryl halide (0.1 mmol, 1.0 eq.), base (4.0 eq.), tetrabutylammonium bromide (1.0 eq.), 1 (1 mol%) and methyl acrylate (6.0 eq.) were placed in an oven-dried and N2-purged flask. The respective solvents (2 mL) were added via a septum and the reaction mixture was stirred at the respective temperature for up to 24 h. Subsequently, the mixture was extracted with chloroform (20 mL) and washed with water (50 mL). The solvent was evaporated under reduced pressure, and the residue was subjected to column-chromatographic separation (silica gel 60, hexane/ethyl acetate, 2:1 as eluent).
Procedure for the Mizoroki–Heck coupling with a catalyst loading of 1 ppm
Complex 1 (0.3 mg, 3.85 × 10−7 mol) was dissolved in NMP (10 mL) and 100 µL of this solution was used (3.85 × 10−9 mol). Phenyliodide (1.155 g, 5.66 × 10−3 mol), K2CO3 (3.13 g), TBABr (1.82 g, 5.66 × 10−3 mol) and methyl acrylate (2.92 g, 3.39 × 10−2 mol) were mixed in NMP (5 mL) and stirred at 100 °C. The solvent was evaporated under reduced pressure and the residue was subjected to a column-chromatographic separation (silica gel 60, hexane/ethyl acetate, 2:1 as eluent) to yield the product as a yellow solid (900 mg, 98%).