Prediction of Sensor Ability Based on Chemical Formula: Possible Approaches and Pitfalls
Abstract
:1. Introduction
2. Results and Discussion
2.1. Levenshtein Distance and Levenshtein Ratio
2.2. Tokenization and Vectorization
2.3. Fingerprint Similarity Methods
2.4. Pitfalls of the Methods of Finding Similarities between SMILES
- Incomplete database: if the database contains no entries about sensors for specific cations or a specific class of chemical compounds considered promising for fluorescent sensing of metal ions, no method can return reliable predictions with high similarity metrics;
- Erroneous entries in the database due to mistakes made by individuals filling the database (e.g., typos in SMILES or misplacement/swapping of cations from different lines);
- Erroneous entries in the database due to mistakes/fraud committed by researchers whose papers served as a source. For example, there may be false claims about the indicator ability of certain compounds towards specific cations or typos in chemical formulas of sensor compounds, etc.
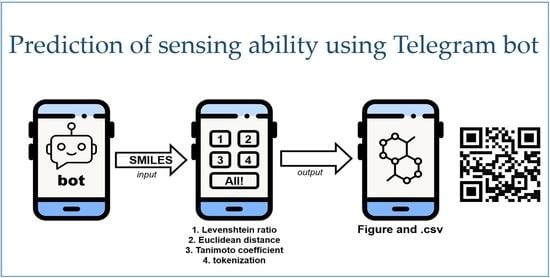
2.5. Program for Prediction of the Sensing Ability of Organic Compounds towards Cations
3. Materials and Methods
3.1. Database
3.2. Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonkaya, Ö.; Soylukan, C.; Pamuk Algi, M.; Algi, F. Aza-BODIPY-Based Fluorescent and Colorimetric Sensors and Probes. COS 2023, 20, 20–60. [Google Scholar] [CrossRef]
- Kikuchi, K.; Adair, L.D.; Lin, J.; New, E.J.; Kaur, A. Photochemical Mechanisms of Fluorophores Employed in Single-Molecule Localization Microscopy. Angew. Chem. Int. Ed. 2023, 62, e202204745. [Google Scholar] [CrossRef]
- Yu, W.; Xu, X.; Jin, K.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Genetically Encoded Biosensors for Microbial Synthetic Biology: From Conceptual Frameworks to Practical Applications. Biotechnol. Adv. 2023, 62, 108077. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Li, X.; Fan, Y.; Li, J.; Lu, K.; Wen, H.; Ren, J. Recent Advances of Fluorescent Sensors for Bacteria Detection-A Review. Talanta 2023, 254, 124133. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Gao, W.; Tang, B. Recent Advancement in Graphene Quantum Dots Based Fluorescent Sensor: Design, Construction and Bio-Medical Applications. Coord. Chem. Rev. 2023, 478, 214966. [Google Scholar] [CrossRef]
- Jie, B.; Lin, H.; Zhai, Y.; Ye, J.; Zhang, D.; Xie, Y.; Zhang, X.; Yang, Y. Mechanism, Design and Application of Fluorescent Recognition Based on Metal Organic Frameworks in Pollutant Detection. Chem. Eng. J. 2023, 454, 139931. [Google Scholar] [CrossRef]
- Tammina, S.K.; Khan, A.; Rhim, J.-W. Advances and Prospects of Carbon Dots for Microplastic Analysis. Chemosphere 2023, 313, 137433. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; He, T.; Wang, G.-M. Zirconium-Based Metal-Organic Frameworks for Fluorescent Sensing. Coord. Chem. Rev. 2023, 476, 214930. [Google Scholar] [CrossRef]
- Chen, S.-H.; Jiang, K.; Xiao, Y.; Cao, X.-Y.; Arulkumar, M.; Wang, Z.-Y. Recent Endeavors on Design, Synthesis, Fluorescence Mechanisms and Applications of Benzazole-Based Molecular Probes toward Miscellaneous Species. Dyes Pigments 2020, 175, 108157. [Google Scholar] [CrossRef]
- Lu, X.-L.; He, W. Research Advances in Excited State Intramolecular Proton Transfer Fluorescent Probes Based on Combined Fluorescence Mechanism. Chin. J. Anal. Chem. 2021, 49, 184–196. [Google Scholar] [CrossRef]
- Liu, J.; Fan, J.; Zhang, K.; Zhang, Y.; Wang, C.-K.; Lin, L. Perspective for Aggregation-Induced Delayed Fluorescence Mechanism: A QM/MM Study*. Chin. Phys. B 2020, 29, 088504. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.; Han, H.-H.; Bull, S.D.; He, X.-P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-State Intramolecular Proton-Transfer (ESIPT) Based Fluorescence Sensors and Imaging Agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef] [Green Version]
- Gamov, G.A.; Zavalishin, M.N.; Petrova, M.V.; Khokhlova, A.Y.; Gashnikova, A.V.; Kiselev, A.N.; Sharnin, V.A. Interaction of Pyridoxal-Derived Hydrazones with Anions and Co2+, Co3+, Ni2+, Zn2+ Cations. Phys. Chem. Liq. 2021, 59, 666–678. [Google Scholar] [CrossRef]
- Gamov, G.A.; Kiselev, A.N.; Murekhina, A.E.; Zavalishin, M.N.; Aleksandriiskii, V.V.; Kosterin, D.Y. Synthesis, Protolytic Equilibria, and Antimicrobial Action of Nifuroxazide Analogs. J. Mol. Liq. 2021, 341, 116911. [Google Scholar] [CrossRef]
- Weininger, D. SMILES, a Chemical Language and Information System. 1. Introduction to Methodology and Encoding Rules. J. Chem. Inf. Model. 1988, 28, 31–36. [Google Scholar] [CrossRef]
- Weininger, D.; Weininger, A.; Weininger, J.L. SMILES. 2. Algorithm for Generation of Unique SMILES Notation. J. Chem. Inf. Comput. Sci. 1989, 29, 97–101. [Google Scholar] [CrossRef]
- Levenshtein, V.I. Binary Codes Capable of Correcting Deletions, Insertions, and Reversals. Sov. Phys. Dokl. 1966, 10, 707–710. [Google Scholar]
- Shao, J.; Gong, Q.; Yin, Z.; Pan, W.; Pandiyan, S.; Wang, L. S2DV: Converting SMILES to a Drug Vector for Predicting the Activity of Anti-HBV Small Molecules. Brief. Bioinform. 2022, 23, bbab593. [Google Scholar] [CrossRef] [PubMed]
- Schwaller, P.; Laino, T.; Gaudin, T.; Bolgar, P.; Hunter, C.A.; Bekas, C.; Lee, A.A. Molecular Transformer: A Model for Uncertainty-Calibrated Chemical Reaction Prediction. ACS Cent. Sci. 2019, 5, 1572–1583. [Google Scholar] [CrossRef] [Green Version]
- Schwaller, P.; Probst, D.; Vaucher, A.C.; Nair, V.H.; Kreutter, D.; Laino, T.; Reymond, J.-L. Mapping the Space of Chemical Reactions Using Attention-Based Neural Networks. Nat. Mach. Intell. 2021, 3, 144–152. [Google Scholar] [CrossRef]
- Cation_Prediction_Bot. Available online: https://t.me/mol_sim_bot (accessed on 9 March 2023).
- Kumar, A.; Virender; Saini, M.; Mohan, B.; Shayoraj; Kamboj, M. Colorimetric and Fluorescent Schiff Base Sensors for Trace Detection of Pollutants and Biologically Significant Cations: A Review (2010–2021). Microchem. J. 2022, 181, 107798. [Google Scholar] [CrossRef]
- Sahoo, S.K. Chromo-Fluorogenic Sensing Using Vitamin B6 Cofactors and Their Derivatives: A Review. New J. Chem. 2021, 45, 8874–8897. [Google Scholar] [CrossRef]
- Berhanu, A.L.; Gaurav; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kumar, V.; Kim, K.-H. A Review of the Applications of Schiff Bases as Optical Chemical Sensors. TrAC Trends Anal. Chem. 2019, 116, 74–91. [Google Scholar] [CrossRef]
- Park, J.-K.; Shin, J.; Jang, S.; Seol, M.-L.; Kang, J.; Choi, S.; Eom, H.; Kwon, O.; Park, S.; Noh, D.-Y.; et al. Rational Design of Fluorescent/Colorimetric Chemosensors for Detecting Transition Metal Ions by Varying Functional Groups. Inorganics 2022, 10, 189. [Google Scholar] [CrossRef]
- De Acha, N.; Elosúa, C.; Corres, J.; Arregui, F. Fluorescent Sensors for the Detection of Heavy Metal Ions in Aqueous Media. Sensors 2019, 19, 599. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Gao, C.-Y.; Liu, J.; Dong, D. Recent Developments in Rhodamine Salicylidene Hydrazone Chemosensors. Anal. Methods 2016, 8, 2863–2871. [Google Scholar] [CrossRef]
- Wan, H.; Xu, Q.; Gu, P.; Li, H.; Chen, D.; Li, N.; He, J.; Lu, J. AIE-Based Fluorescent Sensors for Low Concentration Toxic Ion Detection in Water. J. Hazard. Mater. 2021, 403, 123656. [Google Scholar] [CrossRef]
- Su, X.; Aprahamian, I. Hydrazone-Based Switches, Metallo-Assemblies and Sensors. Chem. Soc. Rev. 2014, 43, 1963. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Chen, X.; Almahri, A.; Allehyani, E.S.; Alhumaydhi, F.A.; Ibrahim, M.M.; Ali, S. Recent Developments in Fluorescent and Colorimetric Chemosensors Based on Schiff Bases for Metallic Cations Detection: A Review. J. Environ. Chem. Eng. 2021, 9, 106381. [Google Scholar] [CrossRef]
- Dutta, M.; Das, D. Recent Developments in Fluorescent Sensors for Trace-Level Determination of Toxic-Metal Ions. TrAC Trends Anal. Chem. 2012, 32, 113–132. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Paper-Based Fluorescence Chemosensors for Metal Ion Detection in Biological and Environmental Samples. BioChip J. 2021, 15, 216–232. [Google Scholar] [CrossRef]
- Prodi, L.; Ballardini, R.; Gandolfi, M.T.; Roversi, R. A Simple Fluorescent Chemosensor for Alkaline-Earth Metal Ions. J. Photochem. Photobiol. A Chem. 2000, 136, 49–52. [Google Scholar] [CrossRef]
- Prodi, L.; Bargossi, C.; Montalti, M.; Zaccheroni, N.; Su, N.; Bradshaw, J.S.; Izatt, R.M.; Savage, P.B. An Effective Fluorescent Chemosensor for Mercury Ions. J. Am. Chem. Soc. 2000, 122, 6769–6770. [Google Scholar] [CrossRef]
- Xia, W.-S.; Schmehl, R.H.; Li, C.-J. A Novel Caesium Selective Fluorescent Chemosensor. Chem. Commun. 2000, 8, 695–696. [Google Scholar] [CrossRef]
- Xia, W.-S.; Schmehl, R.H.; Li, C.-J. A Fluorescent 18-Crown-6 Based Luminescence Sensor for Lanthanide Ions. Tetrahedron 2000, 56, 7045–7049. [Google Scholar] [CrossRef]
- De Santis, G.; Fabbrizzi, L.; Licchelli, M.; Mangano, C.; Sacchi, D.; Sardone, N. A Fluorescent Chemosensor for the Copper(II) Ion. Inorg. Chim. Acta 1997, 257, 69–76. [Google Scholar] [CrossRef]
- Mitchell, K.A.; Brown, R.G.; Yuan, D.; Chang, S.-C.; Utecht, R.E.; Lewis, D.E. A Fluorescent Sensor for Cu2+ at the Sub-Ppm Level. J. Photochem. Photobiol. A Chem. 1998, 115, 157–161. [Google Scholar] [CrossRef]
- Banthia, S.; Sarkar, M.; Samanta, A. Photophysical and Transition Metal Ion Signaling Properties of Some 4-Amino-1,8-Naphthalimide Derivatives. Res. Chem. Intermed. 2005, 31, 25–38. [Google Scholar] [CrossRef]
- Ragos, G.C.; Demertzis, M.A.; Issopoulos, P.B. A High-Sensitive Spectrofluorimetric Method for the Determination of Micromolar Concentrations of Iron(III) in Bovine Liver with 4-Hydroxyquinoline. Il Farmaco 1998, 53, 611–616. [Google Scholar] [CrossRef]
- Obare, S.O.; Murphy, C.J. A Two-Color Fluorescent Lithium Ion Sensor. Inorg. Chem. 2001, 40, 6080–6082. [Google Scholar] [CrossRef]
- Burdette, S.C.; Walkup, G.K.; Spingler, B.; Tsien, R.Y.; Lippard, S.J. Fluorescent Sensors for Zn2+ Based on a Fluorescein Platform: Synthesis, Properties and Intracellular Distribution. J. Am. Chem. Soc. 2001, 123, 7831–7841. [Google Scholar] [CrossRef] [Green Version]
- Klein, G.; Kaufmann, D.; Schürch, S.; Reymond, J.-L. A Fluorescent Metal Sensor Based on Macrocyclic Chelation. Chem. Commun. 2001, 6, 561–562. [Google Scholar] [CrossRef]
- Kawakami, J.; Komai, Y.; Sumori, T.; Fukushi, A.; Shimozaki, K.; Ito, S. Intramolecular Excimer Formation and Complexing Behavior of 1,n-Bis(Naphthalenecarboxy)Oxaalkanes as Fluorescent Chemosensors for Calcium and Barium Ions. J. Photochem. Photobiol. A Chem. 2001, 139, 71–78. [Google Scholar] [CrossRef]
- McFarland, S.A.; Finney, N.S. Fluorescent Chemosensors Based on Conformational Restriction of a Biaryl Fluorophore. J. Am. Chem. Soc. 2001, 123, 1260–1261. [Google Scholar] [CrossRef]
- Kawakami, J.; Bronson, R.T.; Xue, G.; Bradshaw, J.S.; Izatt, R.M.; Savage, P.B. Characterization of Bis-8-Hydroxyquinoline-Armed Diazatrithia-16-Crown-5 and Diazadibenzo-18-Crown-6 Ligands as Fluorescent Chemosensors for Zinc. J. Supramol. Chem. 2001, 1, 221–227. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Bichell, B.; Nolan, C. A Novel Fluorescent Photoinduced Electron Transfer (PET) Sensor for Lithium. Tetrahedron Lett. 2002, 43, 4989–4992. [Google Scholar] [CrossRef]
- Chen, C.-T.; Huang, W.-P. A Highly Selective Fluorescent Chemosensor for Lead Ions. J. Am. Chem. Soc. 2002, 124, 6246–6247. [Google Scholar] [CrossRef]
- Kim, T.W.; Park, J.; Hong, J.-I. Zn2+ Fluorescent Chemosensors and the Influence of Their Spacer Length on Tuning Zn2+ SelectivityElectronic Supplementary Information (ESI) Available: Job Plot, Partial 1H NMR Spectra of Free 3 and the 3–Zn2+ Complex, Ca2+ and Mg2+ Interference for Zn2+ Sensing of 3, Kd Measurements, and Buffer Preparation. See http://www.rsc.org/suppdata/p2/b2/b200462c/. J. Chem. Soc. Perkin Trans. 2002, 2, 923–927. [Google Scholar] [CrossRef]
- Petrat, F.; Weisheit, D.; Lensen, M.; de GROOT, H.; Sustmann, R.; Rauen, U. Selective Determination of Mitochondrial Chelatable Iron in Viable Cells with a New Fluorescent Sensor. Biochem. J. 2002, 362, 137–147. [Google Scholar] [CrossRef]
- Xia, W.-S.; Schmehl, R.H.; Li, C.-J.; Mague, J.T.; Luo, C.-P.; Guldi, D.M. Chemosensors for Lead(II) and Alkali Metal Ions Based on Self-Assembling Fluorescence Enhancement (SAFE). J. Phys. Chem. B 2002, 106, 833–843. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Nieuwenhuyzen, M.; Richard, L.; Thoss, V. Novel Sodium-Selective Fluorescent PET and Optically Based Chemosensors: Towards Na+ Determination in Serum. J. Chem. Soc. Perkin Trans. 2 2002, 1, 141–150. [Google Scholar] [CrossRef]
- Benniston, A.C.; Harriman, A.; Lawrie, D.J.; Mayeux, A.; Rafferty, K.; Russell, O.D. A General Purpose Reporter for Cations: Absorption, Fluorescence and Electrochemical Sensing of Zinc(II). Dalton Trans. 2003, 2, 4762. [Google Scholar] [CrossRef]
- Yang, R.-H.; Chan, W.-H.; Lee, A.W.M.; Xia, P.-F.; Zhang, H.-K.; Ke’An, L. A Ratiometric Fluorescent Sensor for AgI with High Selectivity and Sensitivity. J. Am. Chem. Soc. 2003, 125, 2884–2885. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Wu, S. A Specific Fluorescent Chemosensor for Copper (II) Cation Recognition*. Prog. Nat. Sci. 2003, 13, 201–205. [Google Scholar] [CrossRef]
- Otten, P.A.; London, R.E.; Levy, L.A. 4-Oxo-4 H -Quinolizine-3-Carboxylic Acids as Mg2+ Selective, Fluorescent Indicators. Bioconjugate Chem. 2001, 12, 203–212. [Google Scholar] [CrossRef]
- Nakahara, Y.; Kida, T.; Nakatsuji, Y.; Akashi, M. A Novel Fluorescent Indicator for Ba2+ in Aqueous Micellar SolutionsElectronic Supplementary Information (ESI) Available: Synthesis and Characterisation of 1. Chem. Commun. 2004, 2, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, A.; Momotake, A.; Arai, T. A New Fluorescent Metal Sensor with Two Binding Moieties. Tetrahedron Lett. 2004, 45, 9377–9381. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Soh, J.H.; Yoon, Y.J.; Yoon, J. Highly Effective Fluorescent Sensor for Hg2+ in Aqueous Solution. Supramol. Chem. 2004, 16, 621–624. [Google Scholar] [CrossRef]
- Bolletta, F.; Garelli, A.; Montalti, M.; Prodi, L.; Romano, S.; Zaccheroni, N.; Canovese, L.; Chessa, G.; Santo, C.; Visentin, F. Synthesis, Photophysical Characterisation and Metal Ion Binding Properties of New Ligands Containing Anthracene Chromophores. Inorg. Chim. Acta 2004, 357, 4078–4084. [Google Scholar] [CrossRef] [Green Version]
- Thiagarajan, V.; Selvaraju, C.; Malar, E.J.P.; Ramamurthy, P. A Novel Fluorophore with Dual Fluorescence: Local Excited State and Photoinduced Electron-Transfer-Promoted Charge-Transfer State. ChemPhysChem 2004, 5, 1200–1209. [Google Scholar] [CrossRef]
- Taki, M.; Wolford, J.L.; O’Halloran, T.V. Emission Ratiometric Imaging of Intracellular Zinc: Design of a Benzoxazole Fluorescent Sensor and Its Application in Two-Photon Microscopy. J. Am. Chem. Soc. 2004, 126, 712–713. [Google Scholar] [CrossRef]
- Kawakami, J.; Kimura, H.; Nagaki, M.; Kitahara, H.; Ito, S. Intramolecular Exciplex Formation and Complexing Behavior of 1-(2-Naphthalenecarboxy)-n-(p-Substituted Benzenecarboxy)Oxaalkanes as Fluorescent Chemosensors for Calcium and Barium Ions. J. Photochem. Photobiol. A Chem. 2004, 161, 141–149. [Google Scholar] [CrossRef]
- Chang, C.J.; Jaworski, J.; Nolan, E.M.; Sheng, M.; Lippard, S.J. A Tautomeric Zinc Sensor for Ratiometric Fluorescence Imaging: Application to Nitric Oxide-Induced Release of Intracellular Zinc. Proc. Natl. Acad. Sci. USA 2004, 101, 1129–1134. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Qian, X.; Jia, L. A Highly Selective and Sensitive Fluorescent Chemosensor for Hg2+ in Neutral Buffer Aqueous Solution. J. Am. Chem. Soc. 2004, 126, 2272–2273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, D.X. A Selective, Fluorescent Sensor for Zn2+. ChemPhysChem 2004, 5, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, Q.; Yang, G.; Xiao, C.; Liu, J.; Yang, S.; Ma, J.S. Novel Fluorescent Sensor for Zn(II) Based on Bis(Pyrrol-2-Yl-Methyleneamine) Ligands. Sens. Actuators B Chem. 2004, 99, 511–515. [Google Scholar] [CrossRef]
- Youk, J.-S.; Kim, Y.-H.; Kim, E.-J.; Youn, N.-J.; Chang, S.-K. Hg2+-Selective Chemosensor Derived from 8-Hydroxyquinoline Having Benzothiazole Function in Aqueous Environment. Bull. Korean Chem. Soc. 2004, 25, 869–872. [Google Scholar] [CrossRef]
- Henary, M.M.; Wu, Y.; Fahrni, C.J. Zinc(II)-Selective Ratiometric Fluorescent Sensors Based on Inhibition of Excited-State Intramolecular Proton Transfer. Chem. Eur. J. 2004, 10, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Jaworski, J.; Okamoto, K.-I.; Hayashi, Y.; Sheng, M.; Lippard, S.J. QZ1 and QZ2: Rapid, Reversible Quinoline-Derivatized Fluoresceins for Sensing Biological Zn(II). J. Am. Chem. Soc. 2005, 127, 16812–16823. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.; Albers, A.E.; Wong, A.P.; Chang, C.J. Screening Mercury Levels in Fish with a Selective Fluorescent Chemosensor. J. Am. Chem. Soc. 2005, 127, 16030–16031. [Google Scholar] [CrossRef]
- Shao, N.; Zhang, Y.; Cheung, S.; Yang, R.; Chan, W.; Mo, T.; Li, K.; Liu, F. Copper Ion-Selective Fluorescent Sensor Based on the Inner Filter Effect Using a Spiropyran Derivative. Anal. Chem. 2005, 77, 7294–7303. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kohno, Y.; Hirai, T. Bis-Azamacrocyclic Anthracene as a Fluorescent Chemosensor for Cations in Aqueous Solution. J. Phys. Chem. B 2005, 109, 19139–19147. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.-J.; Wong, W.-Y.; Guo, J.-P.; Wong, W.-K.; Li, Z.-Y. Dipyrrolylquinoxaline-Bridged Schiff Bases: A New Class of Fluorescent Sensors for Mercury(II). Dalton Trans. 2005, 19, 3235–3240. [Google Scholar] [CrossRef]
- Bricks, J.L.; Kovalchuk, A.; Trieflinger, C.; Nofz, M.; Büschel, M.; Tolmachev, A.I.; Daub, J.; Rurack, K. On the Development of Sensor Molecules That Display FeIII-Amplified Fluorescence. J. Am. Chem. Soc. 2005, 127, 13522–13529. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Zhu, D. A Sensitive and Selective “Turn on” Fluorescent Chemosensor for Hg(II) Ion Based on a New Pyrene–Thymine Dyad. Anal. Chim. Acta 2005, 549, 10–13. [Google Scholar] [CrossRef]
- Yang, L.; McRae, R.; Henary, M.M.; Patel, R.; Lai, B.; Vogt, S.; Fahrni, C.J. Imaging of the Intracellular Topography of Copper with a Fluorescent Sensor and by Synchrotron X-Ray Fluorescence Microscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 11179–11184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Peng, X.; Wu, Y.; Lu, E.; Hou, J.; Zhang, H.; Zhang, R.; Fu, X. A New PET Fluorescent Sensor for Zn2+. J. Lumin. 2005, 114, 125–130. [Google Scholar] [CrossRef]
- Komatsu, K.; Kikuchi, K.; Kojima, H.; Urano, Y.; Nagano, T. Selective Zinc Sensor Molecules with Various Affinities for Zn2+, Revealing Dynamics and Regional Distribution of Synaptically Released Zn2+ in Hippocampal Slices. J. Am. Chem. Soc. 2005, 127, 10197–10204. [Google Scholar] [CrossRef]
- Sankaran, N.B.; Nishizawa, S.; Watanabe, M.; Uchida, T.; Teramae, N. Designing Ratiometric Fluorescent Sensors for Alkali Metal Ions from Simple PET Sensors by Controlling Spacer Length. J. Mater. Chem. 2005, 15, 2755. [Google Scholar] [CrossRef]
- Sankaran, N.B.; Mandal, P.K.; Bhattacharya, B.; Samanta, A. Fluorescence Response of Mono-and Tetraazacrown Derivatives of 4-Aminophthalimide with and without Some Transition and Post Transition Metal Ions. J. Mater. Chem. 2005, 15, 2854. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Jang, Y.J.; Lee, Y.J.; Kim, K.M.; Seo, M.S.; Nam, W.; Yoon, J. A Highly Selective Fluorescent Chemosensor for Pb2+. J. Am. Chem. Soc. 2005, 127, 10107–10111. [Google Scholar] [CrossRef] [PubMed]
- Ros-Lis, J.V.; Marcos, M.D.; Mártinez-Máñez, R.; Rurack, K.; Soto, J. A Regenerative Chemodosimeter Based on Metal-Induced Dye Formation for the Highly Selective and Sensitive Optical Determination of Hg2+ Ions. Angew. Chem. Int. Ed. 2005, 44, 4405–4407. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.M. A Tryptophan-Containing Open-Chain Framework for Tuning a High Selectivity for Ca2+ and 13C NMR Observation of a Ca2+ −Indole Interaction in Aqueous Solution. J. Am. Chem. Soc. 2005, 127, 3527–3530. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, Y.; Qian, X.; Cui, J.; Cui, D. Ratiometric and Selective Fluorescent Sensor for CuII Based on Internal Charge Transfer (ICT). Org. Lett. 2005, 7, 889–892. [Google Scholar] [CrossRef]
- Mikata, Y.; Wakamatsu, M.; Yano, S. Tetrakis(2-Quinolinylmethyl)Ethylenediamine (TQEN) as a New Fluorescent Sensor for Zinc. Dalton Trans. 2005, 3, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Chan, W.-H.; He, Y.-B. Selective Complexation of Metals with Isoxazolidine-Containing Fluorophores. Tetrahedron Lett. 2005, 46, 173–176. [Google Scholar] [CrossRef]
- Zhou, L.-L.; Sun, H.; Zhang, X.-H.; Wu, S.-K. An Effective Fluorescent Chemosensor for the Detection of Copper(II). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 61–65. [Google Scholar] [CrossRef]
- Mei, Y.; Bentley, P.A. A Ratiometric Fluorescent Sensor for Zn2+ Based on Internal Charge Transfer (ICT). Bioorg. Med. Chem. Lett. 2006, 16, 3131–3134. [Google Scholar] [CrossRef]
- Nolan, E.M.; Ryu, J.W.; Jaworski, J.; Feazell, R.P.; Sheng, M.; Lippard, S.J. Zinspy Sensors with Enhanced Dynamic Range for Imaging Neuronal Cell Zinc Uptake and Mobilization. J. Am. Chem. Soc. 2006, 128, 15517–15528. [Google Scholar] [CrossRef] [Green Version]
- McCarroll, M.E.; Shi, Y.; Harris, S.; Puli, S.; Kimaru, I.; Xu, R.; Wang, L.; Dyer, D.J. Computational Prediction and Experimental Evaluation of a Photoinduced Electron-Transfer Sensor. J. Phys. Chem. B 2006, 110, 22991–22994. [Google Scholar] [CrossRef]
- Park, M.S.; Swamy, K.M.K.; Lee, Y.J.; Lee, H.N.; Jang, Y.J.; Moon, Y.H.; Yoon, J. A New Acridine Derivative as a Fluorescent Chemosensor for Zinc Ions in an 100% Aqueous Solution: A Comparison of Binding Property with Anthracene Derivative. Tetrahedron Lett. 2006, 47, 8129–8132. [Google Scholar] [CrossRef]
- Wu, J.-S.; Wang, P.-F.; Zhang, X.-H.; Wu, S.-K. Novel Fluorescent Sensor for Detection of Cu(II) in Aqueous Solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 749–752. [Google Scholar] [CrossRef]
- Xu, Z.; Qian, X.; Cui, J.; Zhang, R. Exploiting the Deprotonation Mechanism for the Design of Ratiometric and Colorimetric Zn2+ Fluorescent Chemosensor with a Large Red-Shift in Emission. Tetrahedron 2006, 62, 10117–10122. [Google Scholar] [CrossRef]
- Kulatilleke, C.P.; de Silva, S.A.; Eliav, Y. A Coumarin Based Fluorescent Photoinduced Electron Transfer Cation Sensor. Polyhedron 2006, 25, 2593–2596. [Google Scholar] [CrossRef]
- Wen, G.-T.; Zhu, M.-Z.; Wang, Z.; Meng, X.-M.; Hu, H.-Y.; Guo, Q.-X. Studies on the Transition Metal Ion Induced Fluorescence Enhancement of 1,8-Naphthalimide Derivatives. Chin. J. Chem. 2006, 24, 1230–1237. [Google Scholar] [CrossRef]
- Goldsmith, C.R.; Lippard, S.J. Analogues of Zinpyr-1 Provide Insight into the Mechanism of Zinc Sensing. Inorg. Chem. 2006, 45, 6474–6478. [Google Scholar] [CrossRef]
- Wu, D.-Y.; Xie, L.-X.; Zhang, C.-L.; Duan, C.-Y.; Zhao, Y.-G.; Guo, Z.-J. Quinoline-Based Molecular Clips for Selective Fluorescent Detection of Zn2+. Dalton Trans. 2006, 29, 3528–3533. [Google Scholar] [CrossRef]
- He, Q.; Miller, E.W.; Wong, A.P.; Chang, C.J. A Selective Fluorescent Sensor for Detecting Lead in Living Cells. J. Am. Chem. Soc. 2006, 128, 9316–9317. [Google Scholar] [CrossRef]
- Salman, H.; Tal, S.; Chuvilov, Y.; Solovey, O.; Abraham, Y.; Kapon, M.; Suwinska, K.; Eichen, Y. Sensitive and Selective PET-Based Diimidazole Luminophore for ZnII Ions: A Structure−Activity Correlation. Inorg. Chem. 2006, 45, 5315–5320. [Google Scholar] [CrossRef]
- Kovbasyuk, L.; Krämer, R. A Selective Fluorescent Sensor for Cu2+ and Its Immobilization on CPG Beads. Inorg. Chem. Commun. 2006, 9, 586–590. [Google Scholar] [CrossRef]
- Wang, J.; Qian, X.; Cui, J. Detecting Hg2+ Ions with an ICT Fluorescent Sensor Molecule: Remarkable Emission Spectra Shift and Unique Selectivity. J. Org. Chem. 2006, 71, 4308–4311. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Bricks, J.L.; Resch-Genger, U.; Spieles, M.; Rettig, W. CT-Operated Bifunctional Fluorescent Probe Based on a Pretwisted Donor–Donor–Biphenyl. J. Fluoresc. 2006, 16, 337–348. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Yang, G.-Q.; Zhu, L.-N.; Chen, Q.-Q.; Ma, J.-S. A Potential Fluorescent Sensor for Zn2+ Based on a Selective Bis-9-Anthryldiamine Ligand Operating in Buffer. Sens. Actuators B Chem. 2006, 114, 995–1000. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Ma, J.S.; Yang, G. Ratiometric Zn2+ Sensor and Strategy for Hg2+ Selective Recognition by Central Metal Ion Replacement. Inorg. Chem. 2006, 45, 3140–3142. [Google Scholar] [CrossRef]
- Qi, X.; Jun, E.J.; Xu, L.; Kim, S.-J.; Joong Hong, J.S.; Yoon, Y.J.; Yoon, J. New BODIPY Derivatives as OFF−ON Fluorescent Chemosensor and Fluorescent Chemodosimeter for Cu2+: Cooperative Selectivity Enhancement toward Cu2+. J. Org. Chem. 2006, 71, 2881–2884. [Google Scholar] [CrossRef]
- Nolan, E.M.; Racine, M.E.; Lippard, S.J. Selective Hg(II) Detection in Aqueous Solution with Thiol Derivatized Fluoresceins. Inorg. Chem. 2006, 45, 2742–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.-M.; Zhu, M.-Z.; Liu, L.; Guo, Q.-X. Novel Highly Selective Fluorescent Chemosensors for Zn(II). Tetrahedron Lett. 2006, 47, 1559–1562. [Google Scholar] [CrossRef]
- Goldsmith, C.R.; Lippard, S.J. 6-Methylpyridyl for Pyridyl Substitution Tunes the Properties of Fluorescent Zinc Sensors of the Zinpyr Family. Inorg. Chem. 2006, 45, 555–561. [Google Scholar] [CrossRef]
- Zeng, L.; Miller, E.W.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. A Selective Turn-On Fluorescent Sensor for Imaging Copper in Living Cells. J. Am. Chem. Soc. 2006, 128, 10–11. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Kim, S.-K.; Jun, E.-J.; Xu, L.; Kim, S.-J.; Yoon, J.Y. A New BODIPY Derivative Bearing Piperazine Group. Bull. Korean Chem. Soc. 2007, 28, 2231–2234. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wu, Y.; Cheng, Y.; Yang, H.; Li, F.; Yang, P.; Huang, C. A Ratiometric Fluorescent Sensor for Zinc(II) with High Selectivity. Inorg. Chem. Commun. 2007, 10, 1413–1415. [Google Scholar] [CrossRef]
- Wang, H.-H.; Gan, Q.; Wang, X.-J.; Xue, L.; Liu, S.-H.; Jiang, H. A Water-Soluble, Small Molecular Fluorescent Sensor with Femtomolar Sensitivity for Zinc Ion. Org. Lett. 2007, 9, 4995–4998. [Google Scholar] [CrossRef]
- Komatsu, K.; Urano, Y.; Kojima, H.; Nagano, T. Development of an Iminocoumarin-Based Zinc Sensor Suitable for Ratiometric Fluorescence Imaging of Neuronal Zinc. J. Am. Chem. Soc. 2007, 129, 13447–13454. [Google Scholar] [CrossRef] [PubMed]
- Young, S. Selective Fluorescent Hg(II) Detection in Aqueous Solutions with a Dye Intermediate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 705–709. [Google Scholar] [CrossRef]
- Sumalekshmy, S.; Henary, M.M.; Siegel, N.; Lawson, P.V.; Wu, Y.; Schmidt, K.; Brédas, J.-L.; Perry, J.W.; Fahrni, C.J. Design of Emission Ratiometric Metal-Ion Sensors with Enhanced Two-Photon Cross Section and Brightness. J. Am. Chem. Soc. 2007, 129, 11888–11889. [Google Scholar] [CrossRef]
- Meng, X.M.; Zhu, M.Z.; Guo, Q.X. A Novel Highly Selective Fluorescent Chemosensor for Hg(II) in Fully Aqueous Media. Chin. Chem. Lett. 2007, 18, 1209–1212. [Google Scholar] [CrossRef]
- Yoon, S.; Miller, E.W.; He, Q.; Do, P.H.; Chang, C.J. A Bright and Specific Fluorescent Sensor for Mercury in Water, Cells, and Tissue. Angew. Chem. Int. Ed. 2007, 46, 6658–6661. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.H.; Swamy, K.M.K.; Kim, S.K.; Kim, S.; Lee, S.-H.; Yoon, J. Rhodamine Urea Derivatives as Fluorescent Chemosensors for Hg2+. Tetrahedron Lett. 2007, 48, 5966–5969. [Google Scholar] [CrossRef]
- Mu, H.; Gong, R.; Ma, Q.; Sun, Y.; Fu, E. A Novel Colorimetric and Fluorescent Chemosensor: Synthesis and Selective Detection for Cu2+ and Hg2+. Tetrahedron Lett. 2007, 48, 5525–5529. [Google Scholar] [CrossRef]
- Zhang, X.; Shiraishi, Y.; Hirai, T. A New Rhodamine-Based Fluorescent Chemosensor for Transition Metal Cations Synthesized by One-Step Facile Condensation. Tetrahedron Lett. 2007, 48, 5455–5459. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I.; Soumillion, J.-P.; Bojinov, V. A New Fluorosensor Based on Bis-1,8-Naphthalimide for Metal Cations and Protons. J. Photochem. Photobiol. A Chem. 2007, 189, 192–197. [Google Scholar] [CrossRef]
- Xu, S.; Li, W.; Chen, K.-C. Naphthalimide as Highly Selective Fluorescent Sensor for Ag+Ions. Chin. J. Chem. 2007, 25, 778–783. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Li, M.; Yu, M.; Li, F.; Li, L.; Zhu, M.; Zhang, J.; Yi, T.; Huang, C. A Selective Turn-on Fluorescent Sensor for FeIII and Application to Bioimaging. Tetrahedron Lett. 2007, 48, 3709–3712. [Google Scholar] [CrossRef]
- Peng, R.; Wang, F.; Sha, Y. Synthesis of 5-Dialkyl(Aryl)Aminomethyl-8-Hydroxyquinoline Dansylates as Selective Fluorescent Sensors for Fe3+. Molecules 2007, 12, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Chovelon, J.-M.; Grabchev, I. A Novel Fluorescent Sensor for Metal Cations and Protons Based of Bis-1,8-Naphthalimide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 87–91. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, M.H.; Choe, J.-I.; No, K.T.; Chang, S.-K. Cyclams Bearing Diametrically Disubstituted Pyrenes as Cu2+- and Hg2+-Selective Fluoroionophores. J. Org. Chem. 2007, 72, 3550–3553. [Google Scholar] [CrossRef]
- Peng, X.; Du, J.; Fan, J.; Wang, J.; Wu, Y.; Zhao, J.; Sun, S.; Xu, T. A Selective Fluorescent Sensor for Imaging Cd2+ in Living Cells. J. Am. Chem. Soc. 2007, 129, 1500–1501. [Google Scholar] [CrossRef]
- Dennis, A.E.; Smith, R.C. “Turn-on” Fluorescent Sensor for the Selective Detection of Zinc Ion by a Sterically-Encumbered Bipyridyl-Based Receptor. Chem. Commun. 2007, 44, 4641–4643. [Google Scholar] [CrossRef]
- Parkesh, R.; Clive Lee, T.; Gunnlaugsson, T. Highly Selective 4-Amino-1,8-Naphthalimide Based Fluorescent Photoinduced Electron Transfer (PET) Chemosensors for Zn(II) under Physiological PH Conditions. Org. Biomol. Chem. 2007, 5, 310–317. [Google Scholar] [CrossRef]
- Bojinov, V.B.; Panova, I.P.; Chovelon, J.-M. Novel Blue Emitting Tetra-and Pentamethylpiperidin-4-Yloxy-1,8-Naphthalimides as Photoinduced Electron Transfer Based Sensors for Transition Metal Ions and Protons. Sens. Actuators B Chem. 2008, 135, 172–180. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, N.; Mulrooney, R.C.; Callan, J.F. A Ratiometric Fluorescent Probe for Magnesium Employing Excited State Intramolecular Proton Transfer. Tetrahedron Lett. 2008, 49, 6690–6692. [Google Scholar] [CrossRef]
- Kim, J.; Morozumi, T.; Nakamura, H. Control between TICT and PET Using Chemical Modification of N-Phenyl-9-Anthracenecarboxamide and Its Application to a Crown Ether Type Chemosensor. Tetrahedron 2008, 64, 10735–10740. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sumiya, S.; Kohno, Y.; Hirai, T. A Rhodamine–Cyclen Conjugate as a Highly Sensitive and Selective Fluorescent Chemosensor for Hg(II). J. Org. Chem. 2008, 73, 8571–8574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, Y.; Qian, X. A Ratiometric Fluorescent Probe Based on FRET for Imaging Hg2+ Ions in Living Cells. Angew. Chem. Int. Ed. 2008, 47, 8025–8029. [Google Scholar] [CrossRef]
- Taki, M.; Desaki, M.; Ojida, A.; Iyoshi, S.; Hirayama, T.; Hamachi, I.; Yamamoto, Y. Fluorescence Imaging of Intracellular Cadmium Using a Dual-Excitation Ratiometric Chemosensor. J. Am. Chem. Soc. 2008, 130, 12564–12565. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Fan, J.; Peng, X.; Li, H.; Wang, J.; Sun, S. Highly Selective and Anions Controlled Fluorescent Sensor for Hg2+ in Aqueous Environment. J. Fluoresc. 2008, 18, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Su, J.; Ma, X.; Tian, H. An Efficient Multiple-Mode Molecular Logic System for PH, Solvent Polarity, and Hg2+ Ions. Tetrahedron 2008, 64, 8515–8521. [Google Scholar] [CrossRef]
- Huang, C.; Peng, X.; Lin, Z.; Fan, J.; Ren, A.; Sun, D. A Highly Selective and Sensitive Two-Photon Chemosensor for Silver Ion Derived from 3,9-Dithia-6-Azaundecane. Sens. Actuators B Chem. 2008, 133, 113–117. [Google Scholar] [CrossRef]
- Luo, H.-Y.; Zhang, X.-B.; He, C.-L.; Shen, G.-L.; Yu, R.-Q. Synthesis of Dipicolylamino Substituted Quinazoline as Chemosensor for Cobalt(II) Recognition Based on Excited-State Intramolecular Proton Transfer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 337–342. [Google Scholar] [CrossRef]
- Xue, L.; Wang, H.-H.; Wang, X.-J.; Jiang, H. Modulating Affinities of Di-2-Picolylamine (DPA)-Substituted Quinoline Sensors for Zinc Ions by Varying Pendant Ligands. Inorg. Chem. 2008, 47, 4310–4318. [Google Scholar] [CrossRef]
- Iyoshi, S.; Taki, M.; Yamamoto, Y. Rosamine-Based Fluorescent Chemosensor for Selective Detection of Silver(I) in an Aqueous Solution. Inorg. Chem. 2008, 47, 3946–3948. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; He, Q.; Chang, C.J. Preparation and Use of Leadfluor-1, a Synthetic Fluorophore for Live-Cell Lead Imaging. Nat. Protoc. 2008, 3, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Peng, R.; Sha, Y. Selective Dendritic Fluorescent Sensors for Zn(II). Molecules 2008, 13, 922–930. [Google Scholar] [CrossRef]
- Kim, J.; Morozumi, T.; Kurumatani, N.; Nakamura, H. Novel Chemosensor for Alkaline Earth Metal Ion Based on 9-Anthryl Aromatic Amide Using a Naphthalene as a TICT Control Site and Intramolecular Energy Transfer Donor. Tetrahedron Lett. 2008, 49, 1984–1987. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.Y.; Yoon, S.; Kim, J.S. FRET-Derived Ratiometric Fluorescence Sensor for Cu2+. Tetrahedron 2008, 64, 1294–1300. [Google Scholar] [CrossRef]
- Duan, L.; Xu, Y.; Qian, X. Highly Sensitive and Selective Pd2+ Sensor of Naphthalimide Derivative Based on Complexation with Alkynes and Thio-Heterocycle. Chem. Commun. 2008, 47, 6339–6341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, Y.; Yang, X.; Zang, L. Ultraselective Fluorescent Sensing of Hg2+ through Metal Coordination-Induced Molecular Aggregation. Chem. Commun. 2008, 12, 1413–1415. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, X.; Wua, D.; He, C.; Hu, X.; Duan, C. Structural Modification of Rhodamine-Based Sensors toward Highly Selective Mercury Detection in Mixed Organic/Aqueous Media. Dalton Trans. 2009, 47, 10457–10465. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Liu, H.; Wang, Y.; Shen, Z.; Liu, C.; You, X. Experimentation and Theoretic Calculation of a BODIPY Sensor Based on Photoinduced Electron Transfer for Ions Detection. J. Phys. Chem. A 2009, 113, 14081–14086. [Google Scholar] [CrossRef]
- Dodani, S.C.; He, Q.; Chang, C.J. A Turn-On Fluorescent Sensor for Detecting Nickel in Living Cells. J. Am. Chem. Soc. 2009, 131, 18020–18021. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Li, H.; Wu, Y. A Pyrene-Containing Fluorescent Sensor with High Selectivity for Lead(II) Ion in Water with Dual Illustration of Ground-State Dimer. Sens. Actuators B Chem. 2009, 143, 25–29. [Google Scholar] [CrossRef]
- Gao, T.; Lee, K.M.; Yang, S.I. Synthesis and Characterization of Rhodamine Based Pb2+ Selective Fluorescence Sensor. Toxicol. Environ. Health. Sci. 2009, 1, 159–162. [Google Scholar] [CrossRef]
- Younes, A.H.; Zhang, L.; Clark, R.J.; Zhu, L. Fluorescence of 5-Arylvinyl-5′-Methyl-2,2′-Bipyridyl Ligands and Their Zinc Complexes. J. Org. Chem. 2009, 74, 8761–8772. [Google Scholar] [CrossRef] [PubMed]
- Lohani, C.R.; Kim, J.-M.; Lee, K.-H. Facile Synthesis of Anthracene-Appended Amino Acids as Highly Selective and Sensitive Fluorescent Fe3+ Ion Sensors. Bioorg. Med. Chem. Lett. 2009, 19, 6069–6073. [Google Scholar] [CrossRef]
- Goswami, S.; Chakrabarty, R. Fluorescence Sensing of Cu2+ within a Pseudo 18-Crown-6 Cavity. Tetrahedron Lett. 2009, 50, 5910–5913. [Google Scholar] [CrossRef]
- Mameli, M.; Aragoni, M.C.; Arca, M.; Atzori, M.; Bencini, A.; Bazzicalupi, C.; Blake, A.J.; Caltagirone, C.; Devillanova, F.A.; Garau, A.; et al. Synthesis and Coordination Properties of Quinoline Pendant Arm Derivatives of [9]AneN3 and [9]AneN2 S as Fluorescent Zinc Sensors. Inorg. Chem. 2009, 48, 9236–9249. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Chen, Z.-S.; Wu, C.-Y.; Lin, C.-C.; Yang, C.-R.; Yen, Y.-P. Azo Dyes Featuring a Pyrene Unit: New Selective Chromogenic and Fluorogenic Chemodosimeters for Hg(II). Sens. Actuators B Chem. 2009, 142, 280–287. [Google Scholar] [CrossRef]
- Al-Sayah, M.H.; El-Chami, T.M. Spectroscopic Studies on a ‘Turn-on’ Fluorescent Sensor for Transition Metals with Selective ‘Turn-off’ for Mercury(II) Ions. Supramol. Chem. 2009, 21, 650–657. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, F.; Kim, Y.; Kim, S.-J.; Yoon, J. Cu2+-Selective Ratiometric and “Off-On” Sensor Based on the Rhodamine Derivative Bearing Pyrene Group. Org. Lett. 2009, 11, 4442–4445. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, M.R.; Veismohammadi, B.; Hosseini, M.; Norouzi, P. A New Tb3+-Selective Fluorescent Sensor Based on 2-(5-(Dimethylamino)Naphthalen-1-Ylsulfonyl)-N-Henylhydrazinecarbothioamide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Marques, M.M.B.; Lodeiro, C. Synthesis and Characterization of Novel Indole-Containing Half-Crowns as New Emissive Metal Probes. Tetrahedron Lett. 2009, 50, 4930–4933. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.-B.; Han, Z.-X.; Qiao, L.; Li, C.-Y.; Jian, L.-X.; Shen, G.-L.; Yu, R.-Q. Highly Sensitive and Selective Colorimetric and Off−On Fluorescent Chemosensor for Cu2+ in Aqueous Solution and Living Cells. Anal. Chem. 2009, 81, 7022–7030. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Chen, Z.; Wang, F.; Xue, L.; Jiang, H. High Sensitive Determination of Zinc with Novel Water-Soluble Small Molecular Fluorescent Sensor. Anal. Chim. Acta 2009, 647, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xiong, L.; Liu, H.; Yu, M.; Shen, Z.; Li, F.; You, X. A Highly Selective and Sensitive Fluorescent Turn-on Sensor for Hg2+ and Its Application in Live Cell Imaging. Org. Biomol. Chem. 2009, 7, 2554–2558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, J.; Peng, X. X-Ray Crystallographic and Photophysical Properties of Rhodamine-Based Chemosensor for Fe3+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kaur, N.; Callan, J.F. Incorporation of Siderophore Binding Sites in a Dipodal Fluorescent Sensor for Fe(III). J. Fluoresc. 2009, 19, 649–654. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hong, J.-I. Naphthalimide-Based Fluorescent Zn2+ Chemosensors Showing PET Effect According to Their Linker Length in Water. Tetrahedron Lett. 2009, 50, 2822–2824. [Google Scholar] [CrossRef]
- Roy, P.; Dhara, K.; Manassero, M.; Banerjee, P. Synthesis, Characterization and Selective Fluorescent Zinc(II) Sensing Property of Three Schiff-Base Compounds. Inorg. Chim. Acta 2009, 362, 2927–2932. [Google Scholar] [CrossRef]
- Chen, X.; Jou, M.J.; Lee, H.; Kou, S.; Lim, J.; Nam, S.-W.; Park, S.; Kim, K.-M.; Yoon, J. New Fluorescent and Colorimetric Chemosensors Bearing Rhodamine and Binaphthyl Groups for the Detection of Cu2+. Sens. Actuators B Chem. 2009, 137, 597–602. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Qian, X. A Rhodamine-Based Hg2+ Sensor with High Selectivity and Sensitivity in Aqueous Solution: A NS2 -Containing Receptor. J. Org. Chem. 2009, 74, 2167–2170. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Shi, Y.-T.; Peng, Y.; Zhang, A.-J.; Ma, T.-H.; Dou, W.; Zheng, J.-R. Fluorescent Sensors for Ca2+ and Pb2+ Based on Binaphthyl Derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kaur, N.; Dunn, J.; MacKay, M.; Callan, J.F. A New Fluorescent Chemosensor for Iron(III) Based on the β-Aminobisulfonate Receptor. Tetrahedron Lett. 2009, 50, 953–956. [Google Scholar] [CrossRef]
- Chatterjee, A.; Santra, M.; Won, N.; Kim, S.; Kim, J.K.; Kim, S.B.; Ahn, K.H. Selective Fluorogenic and Chromogenic Probe for Detection of Silver Ions and Silver Nanoparticles in Aqueous Media. J. Am. Chem. Soc. 2009, 131, 2040–2041. [Google Scholar] [CrossRef]
- Dumas, S.; Grabchev, I.; Stoikova, P.; Chauvin, J.; Chovelon, J.-M. Synthesis of Benzanthron Derivatives for Selective Detection by Fluorescence of Copper Ions. J. Photochem. Photobiol. A: Chem. 2009, 201, 237–242. [Google Scholar] [CrossRef]
- Jiao, L.; Li, J.; Zhang, S.; Wei, C.; Hao, E.; Vicente, M.G.H. A Selective Fluorescent Sensor for Imaging Cu2+ in Living Cells. New J. Chem. 2009, 33, 1888–1893. [Google Scholar] [CrossRef]
- Hosseini, M.; Ganjali, M.R.; Abkenar, S.D.; Veismohammadi, B.; Riahl, S.; Norouzi, P.; Salavati-Niasari, M. Highly Selective Ratiometric Fluorescent Sensor for La(III) Ion Based on a New Schiff’s Base. Anal. Lett. 2009, 42, 1029–1040. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Qian, X. A Red-Shift Colorimetric and Fluorescent Sensor for Cu2+ in Aqueous Solution: Unsymmetrical 4,5-Diaminonaphthalimide with N-H Deprotonation Induced by Metal Ions. Org. Biomol. Chem. 2009, 7, 1299–1303. [Google Scholar] [CrossRef]
- Bojinov, V.B.; Georgiev, N.I.; Bosch, P. Design and Synthesis of Highly Photostable Yellow–Green Emitting 1,8-Naphthalimides as Fluorescent Sensors for Metal Cations and Protons. J. Fluoresc. 2009, 19, 127–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Zhu, W.; Zhu, M.; Wu, X.; Tian, H. Near-Infrared Cell-Permeable Hg2+-Selective Ratiometric Fluorescent Chemodosimeters and Fast Indicator Paper for MeHg+ Based on Tricarbocyanines. Chem. Eur. J. 2010, 16, 14424–14432. [Google Scholar] [CrossRef] [PubMed]
- Swamy, K.M.K.; Kim, M.-J.; Jeon, H.-R.; Jung, J.-Y.; Yoon, J.Y. New 7-Hydroxycoumarin-Based Fluorescent Chemosensors for Zn(II) and Cd(II). Bull. Korean Chem. Soc. 2010, 31, 3611–3616. [Google Scholar] [CrossRef] [Green Version]
- Praveen, L.; Reddy, M.L.P.; Varma, R.L. Dansyl-Styrylquinoline Conjugate as Divalent Iron Sensor. Tetrahedron Lett. 2010, 51, 6626–6629. [Google Scholar] [CrossRef]
- Wu, S.-P.; Wang, T.-H.; Liu, S.-R. A Highly Selective Turn-on Fluorescent Chemosensor for Copper(II) Ion. Tetrahedron 2010, 66, 9655–9658. [Google Scholar] [CrossRef]
- Yu, M.R.; Gao, T.; Sun, H.-J.; Yang, S.I. Synthesis and Characterization of Pyrene/Quinoline Based Zn2+ Selective Fluorescent Sensor. Toxicol. Environ. Health Sci. 2010, 2, 158–161. [Google Scholar] [CrossRef]
- Hosseini, M.; Vaezi, Z.; Ganjali, M.R.; Faridbod, F.; Abkenar, S.D.; Salavati-Niasari, M. Selective Recognition of Mercury in Waste Water Based on Fluorescence Enhancement Chemosensor. Sen. Lett. 2010, 8, 807–812. [Google Scholar] [CrossRef]
- Wang, R.-M.; Huang, S.-B.; Zhao, N.; Chen, Z.-N. A New Zn2+ Chemosensor Based on Functionalized 8-Hydroxylquinoline. Inorg. Chem. Commun. 2010, 13, 1432–1434. [Google Scholar] [CrossRef]
- Gao, T.; Yang, S.I. A New Rhodamine-Based Turn-on Fluorescent Chemosensor for Fe3+. Toxicol. Environ. Health Sci. 2010, 2, 73–77. [Google Scholar] [CrossRef]
- Huang, C.; Ren, A.; Feng, C.; Yang, N. Two-Photon Fluorescent Probe for Silver Ion Derived from Twin-Cyano-Stilbene with Large Two-Photon Absorption Cross Section. Sens. Actuators B Chem. 2010, 151, 236–242. [Google Scholar] [CrossRef]
- Zhou, Y.; You, X.-Y.; Fang, Y.; Li, J.-Y.; Liu, K.; Yao, C. A Thiophen-Thiooxorhodamine Conjugate Fluorescent Probe for Detecting Mercury in Aqueous Media and Living Cells. Org. Biomol. Chem. 2010, 8, 4819–4822. [Google Scholar] [CrossRef]
- Ambrosi, G.; Formica, M.; Fusi, V.; Giorgi, L.; Macedi, E.; Micheloni, M.; Paoli, P.; Pontellini, R.; Rossi, P. Efficient Fluorescent Sensors Based on 2,5-Diphenyl [1,3,4] Oxadiazole: A Case of Specific Response to Zn(II) at Physiological PH. Inorg. Chem. 2010, 49, 9940–9948. [Google Scholar] [CrossRef]
- Goswami, S.; Sen, D.; Das, N.K.; Hazra, G. Highly Selective Colorimetric Fluorescence Sensor for Cu2+: Cation-Induced ‘Switching on’ of Fluorescence Due to Excited State Internal Charge Transfer in the Red/near-Infrared Region of Emission Spectra. Tetrahedron Lett. 2010, 51, 5563–5566. [Google Scholar] [CrossRef]
- Lee, D.Y.; Singh, N.; Kim, M.J.; Jang, D.O. Ratiometric Fluorescent Determination of Zn(II): A New Class of Tripodal Receptor Using Mixed Imine and Amide Linkages. Tetrahedron 2010, 66, 7965–7969. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Zhang, B.; Yin, G.; Wang, R. Cell Compatible Fluorescent Chemosensor for Hg2+ with High Sensitivity and Selectivity Based on the Rhodamine Fluorophore. Eur. J. Inorg. Chem. 2010, 2010, 4438–4443. [Google Scholar] [CrossRef]
- Dong, M.; Ma, T.-H.; Zhang, A.-J.; Dong, Y.-M.; Wang, Y.-W.; Peng, Y. A Series of Highly Sensitive and Selective Fluorescent and Colorimetric “off-on” Chemosensors for Cu (II) Based on Rhodamine Derivatives. Dye. Pigment. 2010, 87, 164–172. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Lv, X.; Liu, Y.; Chen, M.; Guo, W. Rhodamine-Based Chemosensor for Hg2+ in Aqueous Solution with a Broad PH Range and Its Application in Live Cell Imaging. Org. Biomol. Chem. 2010, 8, 4143–4147. [Google Scholar] [CrossRef]
- Jung, H.S.; Ko, K.C.; Lee, J.H.; Kim, S.H.; Bhuniya, S.; Lee, J.Y.; Kim, Y.; Kim, S.J.; Kim, J.S. Rationally Designed Fluorescence Turn-On Sensors: A New Design Strategy Based on Orbital Control. Inorg. Chem. 2010, 49, 8552–8557. [Google Scholar] [CrossRef]
- Natali, M.; Soldi, L.; Giordani, S. A Photoswitchable Zn (II) Selective Spiropyran-Based Sensor. Tetrahedron 2010, 66, 7612–7617. [Google Scholar] [CrossRef]
- Zhu, J.-F.; Yuan, H.; Chan, W.-H.; Lee, A.W.M. A Colorimetric and Fluorescent Turn-on Chemosensor Operative in Aqueous Media for Zn2+ Based on a Multifunctionalized Spirobenzopyran Derivative. Org. Biomol. Chem. 2010, 8, 3957–3964. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cao, J.; Chen, C. A Highly Efficient and Selective Turn-on Fluorescent Sensor for Cu2+ Ion. Chin. J. Chem. 2010, 28, 1777–1779. [Google Scholar] [CrossRef]
- Mashraqui, S.H.; Betkar, R.; Chandiramani, M.; Poonia, K.; Quinonero, D.; Frontera, A. New 1,8-Naphthyridine-Based Probes for the Selective Fluorescence Signalling of Toxic Cadmium: Synthesis, Photophysical Studies and Molecular Modelling. Supramol. Chem. 2010, 22, 524–531. [Google Scholar] [CrossRef]
- Wang, X.M.; Yan, H.; Feng, X.L.; Chen, Y. 1-Pyrenecarboxaldehyde Thiosemicarbazone: A Novel Fluorescent Molecular Sensor towards Mercury (II) Ion. Chin. Chem. Lett. 2010, 21, 1124–1128. [Google Scholar] [CrossRef]
- Lin, W.; Cao, X.; Ding, Y.; Yuan, L.; Yu, Q. A Reversible Fluorescent Hg2+ Chemosensor Based on a Receptor Composed of a Thiol Atom and an Alkene Moiety for Living Cell Fluorescence Imaging. Org. Biomol. Chem. 2010, 8, 3618–3620. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Tian, H. Chemodosimeters for Mercury(II) and Methylmercury(I) Based on 2,1,3-Benzothiadiazole. Sens. Actuators B Chem. 2010, 149, 20–27. [Google Scholar] [CrossRef]
- Ciesienski, K.L.; Hyman, L.M.; Derisavifard, S.; Franz, K.J. Toward the Detection of Cellular Copper(II) by a Light-Activated Fluorescence Increase. Inorg. Chem. 2010, 49, 6808–6810. [Google Scholar] [CrossRef] [Green Version]
- Pibiri, I.; Palumbo Piccionello, A.; Calabrese, A.; Buscemi, S.; Vivona, N.; Pace, A. Fluorescent Hg2+ Sensors: Synthesis and Evaluation of a Tren-Based Starburst Molecule Containing Fluorinated 1,2,4-Oxadiazoles. Eur. J. Org. Chem. 2010, 2010, 4549–4553. [Google Scholar] [CrossRef]
- Jung, H.J.; Singh, N.; Lee, D.Y.; Jang, D.O. Single Sensor for Multiple Analytes: Chromogenic Detection of I−and Fluorescent Detection of Fe3+. Tetrahedron Lett. 2010, 51, 3962–3965. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, W.; Xie, Y.; Li, X.; Gao, Y.; Li, F.; Tian, H. Near-IR Core-Substituted Naphthalenediimide Fluorescent Chemosensors for Zinc Ions: Ligand Effects on PET and ICT Channels. Chem. Eur. J. 2010, 16, 8355–8364. [Google Scholar] [CrossRef]
- Staneva, D.; McKena, M.; Bosch, P.; Grabchev, I. Synthesis and Spectroscopic Studies of a New 1,8-Naphthalimide Dyad as Detector for Metal Cations and Protons. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 76, 150–154. [Google Scholar] [CrossRef]
- Xi, P.; Dou, J.; Huang, L.; Xu, M.; Chen, F.; Wu, Y.; Bai, D.; Li, W.; Zeng, Z. A Selective Turn-on Fluorescent Sensor for Cu(II) and Its Application in Imaging in Living Cells. Sens. Actuators B Chem. 2010, 148, 337–341. [Google Scholar] [CrossRef]
- Li, H.-W.; Wang, B.; Dang, Y.-Q.; Li, L.; Wu, Y. A Highly Selective Fluorescent Sensor for Mercury Ions in Aqueous Solution: Detection Based on Target-Induced Aggregation. Sens. Actuators B Chem. 2010, 148, 49–53. [Google Scholar] [CrossRef]
- Bojinov, V.B.; Georgiev, N.I.; Marinova, N.V. Design and Synthesis of Highly Photostable Fluorescence Sensing 1,8-Naphthalimide-Based Dyes Containing s-Triazine UV Absorber and HALS Units. Sens. Actuators B Chem. 2010, 148, 6–16. [Google Scholar] [CrossRef]
- Moon, K.-S.; Yang, Y.-K.; Ji, S.; Tae, J. Aminoxy-Linked Rhodamine Hydroxamate as Fluorescent Chemosensor for Fe3+ in Aqueous Media. Tetrahedron Lett. 2010, 51, 3290–3293. [Google Scholar] [CrossRef]
- Lin, W.-C.; Wu, C.-Y.; Liu, Z.-H.; Lin, C.-Y.; Yen, Y.-P. A New Selective Colorimetric and Fluorescent Sensor for Hg2+ and Cu2+ Based on a Thiourea Featuring a Pyrene Unit. Talanta 2010, 81, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Milewska, M.; Guzow, K.; Wiczk, W. Fluorescent Chemosensors for Metal Ions Based on 3-(2-Benzoxazol-5-Yl)Alanine Skeleton. Open Chem. 2010, 8, 674–686. [Google Scholar] [CrossRef]
- Martínez, R.; Espinosa, A.; Tárraga, A.; Molina, P. A New Bis(Pyrenyl)Azadiene-Based Probe for the Colorimetric and Fluorescent Sensing of Cu(II) and Hg(II). Tetrahedron 2010, 66, 3662–3667. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Wang, Y.-J.; Lin, Y.-J.; Hu, C.-H.; Chen, S.-J.; Chir, J.-L.; Wu, A.-T. A Water-Soluble Ribosyl-Based Fluorescent Sensor for Hg2+ and Cu2+ Ions. Carbohydr. Res. 2010, 345, 956–959. [Google Scholar] [CrossRef]
- Yıldırım, M.; Kaya, İ. Synthesis of a Novel Fluorescent Schiff Base as a Possible Cu(II) Ion Selective Sensor. J. Fluoresc. 2010, 20, 771–777. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, B.; Yang, Z.; Li, T.; Li, Y. A Novel Fluorescent Chemosensor for Zn(II) Based on 1,2-(2′-Oxoquinoline-3′-Yl-Methylideneimino)Ethane. Inorg. Chem. Commun. 2010, 13, 606–608. [Google Scholar] [CrossRef]
- Ma, T.-H.; Zhang, A.-J.; Dong, M.; Dong, Y.-M.; Peng, Y.; Wang, Y.-W. A Simply and Highly Selective “Turn-on” Type Fluorescent Chemosensor for Hg2+ Based on Chiral BINOL-Schiff’s Base Ligand. J. Lumin. 2010, 130, 888–892. [Google Scholar] [CrossRef]
- Tamanini, E.; Flavin, K.; Motevalli, M.; Piperno, S.; Gheber, L.A.; Todd, M.H.; Watkinson, M. Cyclam-Based “Clickates”: Homogeneous and Heterogeneous Fluorescent Sensors for Zn(II). Inorg. Chem. 2010, 49, 3789–3800. [Google Scholar] [CrossRef]
- Han, Z.-X.; Zhang, X.-B.; Li, Z.; Gong, Y.-J.; Wu, X.-Y.; Jin, Z.; He, C.-M.; Jian, L.-X.; Zhang, J.; Shen, G.-L.; et al. Efficient Fluorescence Resonance Energy Transfer-Based Ratiometric Fluorescent Cellular Imaging Probe for Zn2+ Using a Rhodamine Spirolactam as a Trigger. Anal. Chem. 2010, 82, 3108–3113. [Google Scholar] [CrossRef]
- Zhu, M.-Q.; Gu, Z.; Zhang, R.; Xiang, J.-N.; Nie, S. A Stilbene-Based Fluoroionophore for Copper Ion Sensing in Both Reduced and Oxidized Environments. Talanta 2010, 81, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Ganjali, M.R.; Veismohammadi, B.; Norouzi, P.; Alizadeh, K.; Abkenar, S.D. A Novel Ratiometric Fluorescent Yb3+ Sensor Based on a N′-(1-Oxoacenaphthylen-2(1H)-Ylidene)Furan-2-Carbohydrazide as a Suitable Fluorophore. Mater. Sci. Eng. C 2010, 30, 348–351. [Google Scholar] [CrossRef]
- Wan, Y.; Guo, Q.; Wang, X.; Xia, A. Photophysical Properties of Rhodamine Isomers: A Two-Photon Excited Fluorescent Sensor for Trivalent Chromium Cation (Cr3+). Anal. Chim. Acta 2010, 665, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Men, G.; Zhao, L.; Hou, Q.; Jiang, S. Binaphthyl-Derived Salicylidene Schiff Base for Dual-Channel Sensing of Cu, Zn Cations and Integrated Molecular Logic Gates. Sens. Actuators B Chem. 2010, 145, 826–831. [Google Scholar] [CrossRef]
- Hu, Z.-Q.; Yang, X.-D.; Cui, C.-L.; Ding, L.; Lin, C.-S.; Lu, H.-Y.; Wang, L. 1,8-Anthrancene Disulfonamide: A Simple but Highly Sensitive and Selective Fluorescent Chemosensor for Hg2+ in Aqueous Media. Sens. Actuators B Chem. 2010, 145, 61–65. [Google Scholar] [CrossRef]
- Wanichacheva, N.; Watpathomsub, S.; Lee, V.S.; Grudpan, K. Synthesis of a Novel Fluorescent Sensor Bearing Dansyl Fluorophores for the Highly Selective Detection of Mercury (II) Ions. Molecules 2010, 15, 1798–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, D.; Wang, Q.; Li, X.; Schalley, C.A. Nickel(II) and Iron(III) Selective off-on-Type Fluorescence Probes Based on Perylene Tetracarboxylic Diimide. Org. Biomol. Chem. 2010, 8, 1017–1026. [Google Scholar] [CrossRef]
- Bojinov, V.B.; Panova, I.P.; Simeonov, D.B.; Georgiev, N.I. Synthesis and Sensor Activity of Photostable Blue Emitting 1,8-Naphthalimides Containing s-Triazine UV Absorber and HALS Fragments. J. Photochem. Photobiol. A Chem. 2010, 210, 89–99. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, W.; Xu, Y.; Zhang, S.; Zhang, X.; Qian, X. A Thioether-Rich Crown-Based Highly Selective Fluorescent Sensor for Hg2+ and Ag+ in Aqueous Solution. Dalton Trans. 2010, 39, 1316–1320. [Google Scholar] [CrossRef]
- Goswami, S.; Sen, D.; Das, N.K. A New Highly Selective, Ratiometric and Colorimetric Fluorescence Sensor for Cu2+ with a Remarkable Red Shift in Absorption and Emission Spectra Based on Internal Charge Transfer. Org. Lett. 2010, 12, 856–859. [Google Scholar] [CrossRef]
- Lee, D.Y.; Singh, N.; Jang, D.O. A Benzimidazole-Based Single Molecular Multianalyte Fluorescent Probe for the Simultaneous Analysis of Cu2+ and Fe3+. Tetrahedron Lett. 2010, 51, 1103–1106. [Google Scholar] [CrossRef]
- Li, L.; Dang, Y.-Q.; Li, H.-W.; Wang, B.; Wu, Y. Fluorescent Chemosensor Based on Schiff Base for Selective Detection of Zinc(II) in Aqueous Solution. Tetrahedron Lett. 2010, 51, 618–621. [Google Scholar] [CrossRef]
- Xu, Z.; Baek, K.-H.; Kim, H.N.; Cui, J.; Qian, X.; Spring, D.R.; Shin, I.; Yoon, J. Zn2+ -Triggered Amide Tautomerization Produces a Highly Zn2+ -Selective, Cell-Permeable, and Ratiometric Fluorescent Sensor. J. Am. Chem. Soc. 2010, 132, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Mameli, M.; Aragoni, M.C.; Arca, M.; Caltagirone, C.; Demartin, F.; Farruggia, G.; De Filippo, G.; Devillanova, F.A.; Garau, A.; Isaia, F.; et al. A Selective, Nontoxic, OFF-ON Fluorescent Molecular Sensor Based on 8-Hydroxyquinoline for Probing Cd2+ in Living Cells. Chem. A Eur. J. 2010, 16, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-H.; Xue, L.; Qian, Y.-Y.; Jiang, H. Novel Ratiometric Fluorescent Sensor for Silver Ions. Org. Lett. 2010, 12, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, K.; Muramatsu, Y.; Urano, Y.; Terai, T.; Nagano, T. Design and Synthesis of a Highly Sensitive Off–On Fluorescent Chemosensor for Zinc Ions Utilizing Internal Charge Transfer. Chem. Eur. J. 2010, 16, 568–572. [Google Scholar] [CrossRef]
- Hu, Z.-Q.; Lin, C.; Wang, X.-M.; Ding, L.; Cui, C.-L.; Liu, S.-F.; Lu, H.Y. Highly Sensitive and Selective Turn-on Fluorescent Chemosensor for Pb2+ and Hg2+ Based on a Rhodamine–Phenylurea Conjugate. Chem. Commun. 2010, 46, 3765–3767. [Google Scholar] [CrossRef]
- Moro, A.J.; Cywinski, P.J.; Körsten, S.; Mohr, G.J. An ATP Fluorescent Chemosensor Based on a Zn(II)-Complexed Dipicolylaminereceptor Coupled with a Naphthalimidechromophore. Chem. Commun. 2010, 46, 1085–1087. [Google Scholar] [CrossRef]
- Ashokkumar, P.; Ramakrishnan, V.T.; Ramamurthy, P. Photoinduced Electron Transfer (PET) Based Zn2+ Fluorescent Probe: Transformation of Turn-On Sensors into Ratiometric Ones with Dual Emission in Acetonitrile. J. Phys. Chem. A 2011, 115, 14292–14299. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.H.; Pradhan, T.; Lim, C.S.; Cho, B.R.; Bhuniya, S.; Kim, S.; Kim, J.S. Fluorescent Turn-on Zn2+ Sensing in Aqueous and Cellular Media. Sens. Actuators B Chem. 2011, 160, 1489–1493. [Google Scholar] [CrossRef]
- Wang, H.-H.; Xue, L.; Yu, C.-L.; Qian, Y.-Y.; Jiang, H. Rhodamine-Based Fluorescent Sensor for Mercury in Buffer Solution and Living Cells. Dye. Pigment. 2011, 91, 350–355. [Google Scholar] [CrossRef]
- Gong, Z.-L.; Ge, F.; Zhao, B.-X. Novel Pyrazoline-Based Selective Fluorescent Sensor for Zn2+ in Aqueous Media. Sens. Actuators B Chem. 2011, 159, 148–153. [Google Scholar] [CrossRef]
- Wanichacheva, N.; Kumsorn, P.; Sangsuwan, R.; Kamkaew, A.; Lee, V.S.; Grudpan, K. A New Fluorescent Sensor Bearing Three Dansyl Fluorophores for Highly Sensitive and Selective Detection of Mercury(II) Ions. Tetrahedron Lett. 2011, 52, 6133–6136. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Li, B.; Zhang, L.; Song, H.; Jiang, H.; Gao, J. A Cell Compatible Fluorescent Chemosensor for Hg2+ Based on a Novel Rhodamine Derivative That Works as a Molecular Keypad Lock. RSC Adv. 2011, 1, 1294–1300. [Google Scholar] [CrossRef]
- Dai, H.; Liu, F.; Gao, Q.; Fu, T.; Kou, X. A Highly Selective Fluorescent Sensor for Mercury Ion (II) Based on Azathia-Crown Ether Possessing a Dansyl Moiety. Luminescence 2011, 26, 523–530. [Google Scholar] [CrossRef]
- Carney, P.; Lopez, S.; Mickley, A.; Grinberg, K.; Zhang, W.; Dai, Z. Multimode Selective Detection of Mercury by Chiroptical Fluorescent Sensors Based on Methionine/Cysteine. Chirality 2011, 23, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, R.; Parviz, M.; Tamari, E.; Keypour, H.; Golbedaghi, R. Highly Selective Fluorescent Recognition of Zn2+ Based on Naphthalene Macrocyclic Derivative. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Abebe, F.A.; Eribal, C.S.; Ramakrishna, G.; Sinn, E. A ‘Turn-on’ Fluorescent Sensor for the Selective Detection of Cobalt and Nickel Ions in Aqueous Media. Tetrahedron Lett. 2011, 52, 5554–5558. [Google Scholar] [CrossRef]
- Vallejos, S.; Estévez, P.; Ibeas, S.; Muñoz, A.; García, F.C.; Serna, F.; García, J.M. A Selective and Highly Sensitive Fluorescent Probe of Hg2+ in Organic and Aqueous Media: The Role of a Polymer Network in Extending the Sensing Phenomena to Water Environments. Sens. Actuators B Chem. 2011, 157, 686–690. [Google Scholar] [CrossRef]
- Dalapati, S.; Paul, B.K.; Jana, S.; Guchhait, N. Highly Selective and Sensitive Fluorescence Reporter for Toxic Hg(II) Ion by a Synthetic Symmetrical Azine Derivative. Sens. Actuators B Chem. 2011, 157, 615–620. [Google Scholar] [CrossRef]
- Chen, L.; Yang, L.; Li, H.; Gao, Y.; Deng, D.; Wu, Y.; Ma, L. Tridentate Lysine-Based Fluorescent Sensor for Hg(II) in Aqueous Solution. Inorg. Chem. 2011, 50, 10028–10032. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, R.-F.; Xing, S.-K.; Liu, X.-M.; Hu, T.-L.; Bu, X.-H. A Highly Selective On/Off Fluorescence Sensor for Cadmium(II). Inorg. Chem. 2011, 50, 10041–10046. [Google Scholar] [CrossRef]
- Wen, J.; Geng, Z.; Yin, Y.; Wang, Z. A Versatile Water Soluble Fluorescent Probe for Ratiometric Sensing of Hg2+ and Bovine Serum Albumin. Dalton Trans. 2011, 40, 9737–9745. [Google Scholar] [CrossRef] [PubMed]
- Abebe, F.A.; Sinn, E. Fluorescein-Based Fluorescent and Colorimetric Chemosensors for Copper in Aqueous Media. Tetrahedron Lett. 2011, 52, 5234–5237. [Google Scholar] [CrossRef]
- Yang, M.-H.; Thirupathi, P.; Lee, K.-H. Selective and Sensitive Ratiometric Detection of Hg(II) Ions Using a Simple Amino Acid Based Sensor. Org. Lett. 2011, 13, 5028–5031. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, N.; Maity, D.; Govindaraju, T. Reversible Fluorescence Sensing of Zn2+ Based on Pyridine-Constrained Bis (Triazole-Linked Hydroxyquinoline) Sensor. Supramol. Chem. 2011, 23, 703–709. [Google Scholar] [CrossRef]
- Gou, C.; Qin, S.-H.; Wu, H.-Q.; Wang, Y.; Luo, J.; Liu, X.-Y. A Highly Selective Chemosensor for Cu2+ and Al3+ in Two Different Ways Based on Salicylaldehyde Schiff. Inorg. Chem. Commun. 2011, 14, 1622–1625. [Google Scholar] [CrossRef]
- Goswami, S.; Das, N.K.; Aich, K.; Sen, D. A Naphthyridine Based Macrocyclic “Switching on” Fluorescent Receptor for Cadmium. J. Lumin. 2011, 131, 2185–2188. [Google Scholar] [CrossRef]
- Maity, D.; Manna, A.K.; Karthigeyan, D.; Kundu, T.K.; Pati, S.K.; Govindaraju, T. Visible-Near-Infrared and Fluorescent Copper Sensors Based on Julolidine Conjugates: Selective Detection and Fluorescence Imaging in Living Cells. Chem. Eur. J. 2011, 17, 11152–11161. [Google Scholar] [CrossRef]
- Hou, J.-T.; Zhang, Q.-F.; Xu, B.-Y.; Lu, Q.-S.; Liu, Q.; Zhang, J.; Yu, X.-Q. A Novel BINOL-Based Cyclophane via Click Chemistry: Synthesis and Its Applications for Sensing Silver Ions. Tetrahedron Lett. 2011, 52, 4927–4930. [Google Scholar] [CrossRef]
- Hou, C.; Urbanec, A.M.; Cao, H. A Rapid Hg2+ Sensor Based on Aza-15-Crown-5 Ether Functionalized 1,8-Naphthalimide. Tetrahedron Lett. 2011, 52, 4903–4905. [Google Scholar] [CrossRef]
- Tang, L.; Li, F.; Liu, M.; Nandhakumar, R. A New Rhodamine B-Coumarin Fluorochrome for Colorimetric Recognition of Cu2+ and Fluorescent Recognition of Fe3+ in Aqueous Media. Bull. Korean Chem. Soc. 2011, 32, 3400–3404. [Google Scholar] [CrossRef] [Green Version]
- Neupane, L.N.; Thirupathi, P.; Jang, S.; Jang, M.J.; Kim, J.H.; Lee, K.-H. Highly Selectively Monitoring Heavy and Transition Metal Ions by a Fluorescent Sensor Based on Dipeptide. Talanta 2011, 85, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Praveen, L.; Suresh, C.H.; Reddy, M.L.P.; Luxmi Varma, R. Molecular Fluorescent Probe for Zn2+ Based on 2-(2-Nitrostyryl)-8-Methoxyquinoline. Tetrahedron Lett. 2011, 52, 4730–4733. [Google Scholar] [CrossRef]
- Ruan, Y.-B.; Maisonneuve, S.; Xie, J. Highly Selective Fluorescent and Colorimetric Sensor for Hg2+ Based on Triazole-Linked NBD. Dye. Pigment. 2011, 90, 239–244. [Google Scholar] [CrossRef]
- GeorgeGamov/Prediction of Sensing Ability GitLab. Available online: https://gitlab.com/GeorgeGamov/prediction-of-sensing-ability (accessed on 10 February 2023).
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-XTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welcome to Levenshtein’s Documentation!—Levenshtein 0.20.9 Documentation. Available online: https://maxbachmann.github.io/Levenshtein/ (accessed on 9 February 2023).
- RDKit. Available online: http://www.rdkit.org/ (accessed on 9 March 2023).
- Scikit-Learn: Machine Learning in Python—Scikit-Learn 1.2.2 Documentation. Available online: https://scikit-learn.org/stable/ (accessed on 9 March 2023).
- Aiogram. Available online: https://aiogram.dev/ (accessed on 9 March 2023).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarullin, D.N.; Zavalishin, M.N.; Gamov, G.A.; Lukanov, M.M.; Ksenofontov, A.A.; Bumagina, N.A.; Antina, E.V. Prediction of Sensor Ability Based on Chemical Formula: Possible Approaches and Pitfalls. Inorganics 2023, 11, 158. https://doi.org/10.3390/inorganics11040158
Yarullin DN, Zavalishin MN, Gamov GA, Lukanov MM, Ksenofontov AA, Bumagina NA, Antina EV. Prediction of Sensor Ability Based on Chemical Formula: Possible Approaches and Pitfalls. Inorganics. 2023; 11(4):158. https://doi.org/10.3390/inorganics11040158
Chicago/Turabian StyleYarullin, Daniil N., Maksim N. Zavalishin, George A. Gamov, Michail M. Lukanov, Alexander A. Ksenofontov, Natalia A. Bumagina, and Elena V. Antina. 2023. "Prediction of Sensor Ability Based on Chemical Formula: Possible Approaches and Pitfalls" Inorganics 11, no. 4: 158. https://doi.org/10.3390/inorganics11040158