Simultaneous Quantification of Fullerenes C60 and C70 in Organic Solvents by Excitation–Emission Matrix Fluorescence Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
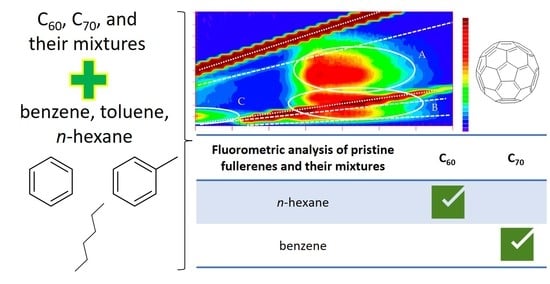
2.1. EEM Features of C60
2.2. EEM Features of C70
2.3. Performance Parameters
2.4. Real Sample Analysis
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Spectrophotometric Quantification of C60 and C70
References
- Devi, N.; Kumar, R.; Chen, Y.-S.; Singh, R.K. Carbon-Based Nanomaterials: Carbon Nanotube, Fullerene, and Carbon Dots. In Nanomaterials; Singh, D.K., Singh, S., Singh, P., Eds.; Springer: Singapore, 2023; pp. 27–57. [Google Scholar]
- Zhang, L.; Xu, K.; Wei, F. Fabrication of electronic switches based on low-dimensional nanomaterials: A review. J. Mater. Sci. 2023, 58, 2087–2110. [Google Scholar] [CrossRef]
- Bernardo, G.; Melle-Franco, M.; Washington, A.L.; Dalgliesh, R.M.; Li, F.; Mendes, A.; Parnell, S.R. Different agglomeration properties of PC 61 BM and PC 71 BM in photovoltaic inks–a spin-echo SANS study. RSC Adv. 2020, 10, 4512–4520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmannova, D.; Borsky, P.; Svadlakova, T.; Borska, L.; Fiala, Z. Carbon Nanoparticles and Their Biomedical Applications. Appl. Sci. 2022, 12, 7865. [Google Scholar] [CrossRef]
- Yi, H.; Zeng, G.; Lai, C.; Huang, D.; Tang, L.; Gong, J.; Chen, M.; Xu, P.; Wang, H.; Cheng, M.; et al. Environment-friendly fullerene separation methods. Chem. Eng. J. 2017, 330, 134–145. [Google Scholar] [CrossRef]
- Astefanei, A.; Nunez, O.; Galceran, M.T. Characterisation and determination of fullerenes: A critical review. Anal. Chim. Acta 2015, 882, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsov, S.I.; Yunusova, D.S.; Yumagulova, R.K.; Miftakhov, M.S.; Kolesov, S.V.; Spivak, S.I.; Kantor, O.G. Quantitative UV Spectrophotometric Analysis of Mixtures of Substituted C60 Fullerenes. J. Appl. Spectrosc. 2015, 82, 644–652. [Google Scholar] [CrossRef]
- Tran, C.D.; Grishko, V.I.; Challa, S. Near-infrared spectrophotometric determination of compositions of fullerene samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 62, 38–41. [Google Scholar] [CrossRef]
- Kimbrell, J.B.; Crittenden, C.M.; Steward, W.J.; Khan, F.A.; Gaquere-Parker, A.C.; Stuart, D.A. Analysis of mixtures of C60and C70by Raman spectrometry. Nanosci. Methods 2014, 3, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Isaacson, C.W.; Kleber, M.; Field, J.A. Quantitative Analysis of Fullerene Nanomaterials in Environmental Systems: A Critical Review. Environ. Sci. Technol. 2009, 43, 6463–6474. [Google Scholar] [CrossRef]
- Isaacson, C.W.; Usenko, C.Y.; Tanguay, R.L.; Field, J.A. Quantification of fullerenes by LC/ESI-MS and its application to in vivo toxicity assays. Anal. Chem. 2007, 79, 9091–9097. [Google Scholar] [CrossRef]
- Chen, Z.; Westerhoff, P.; Herckes, P. Quantification of C60 fullerene concentrations in water. Environ. Toxicol. Chem. 2008, 27, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.M.; Westerhoff, P.; Herckes, P. Detection of fullerenes (C60 and C70) in commercial cosmetics. Environ. Pollut. 2011, 159, 1334–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, R.; Chattopadhyay, S.; Bhattacharya, S. Physicochemical insights in supramolecular interaction of fullerenes C60 and C70 with a monoporphyrin in presence of silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 89, 284–293. [Google Scholar] [CrossRef]
- Williams, R.M.; Verhoeven, J.W. Fluorescence of fullerene-C70 and its quenching by long-range intermolecular electron transfer. Chem. Phys. Lett. 1992, 194, 446–451. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Fang, Y.; Jiang, Y. Fluorescence study of fullerene in organic solvents at room temperature. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 64, 564–567. [Google Scholar] [CrossRef]
- Ma, B.; Sun, Y.-P. Fluorescence spectra and quantum yields of [60]fullerene and [70]fullerene under different solvent conditions. A quantitative examination using a near-infrared-sensitive emission spectrometer. J. Chem. Soc. Perkin Trans. 1996, 2, 2157–2162. [Google Scholar] [CrossRef]
- Catalan, J.; Elguero, J. Fluorescence of fullerenes (C60 and C70). J. Am. Chem. Soc. 1993, 115, 9249–9252. [Google Scholar] [CrossRef]
- Song, J.; Li, F.; Qian, S.; Li, Y.; Peng, W.; Zhou, J.; Yu, Z. Time-Resolved Fluorescence Study of C 60 Solution. Chin. Phys. Lett. 1994, 11, 137. [Google Scholar] [CrossRef]
- Zhang, F.; Fang, Y. Nanostructure origins of C60 fluorescence in pyridine. J. Phys. Chem. B 2006, 110, 9022–9026. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X. On the fluorescence of C60 at room temperature. Sci. China Phys. Mech. Astron. 2010, 53, 95–99. [Google Scholar] [CrossRef]
- Baleizao, C.; Berberan-Santos, M.N. A molecular thermometer based on the delayed fluorescence of C70 dispersed in a polystyrene film. J. Fluoresc. 2006, 16, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baleizao, C.; Berberan-Santos, M.N. Thermally activated delayed fluorescence in fullerenes. Ann. N. Y. Acad. Sci. 2008, 1130, 224–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Lee, M.; Suh, Y.D.; Kim, S.K. Observation of fluorescence emission from solutions of C60 and C70 fullerenes and measurement of their excited-state lifetimes. J. Am. Chem. Soc. 1992, 114, 4429–4430. [Google Scholar] [CrossRef]
- Wild, U.P.; Luond, M.; Meister, E.; Suter, G.W. Total luminescence spectroscopy. J. Lumin. 1988, 40–41, 270–271. [Google Scholar] [CrossRef]
- Weber, G. Enumeration of components in complex systems by fluorescence spectrophotometry. Nature 1961, 190, 27–29. [Google Scholar] [CrossRef]
- Saraswati, T.E.; Setiawan, U.H.; Ihsan, M.R.; Isnaeni, I.; Herbani, Y. The Study of the Optical Properties of C60 Fullerene in Different Organic Solvents. Open Chem. 2019, 17, 1198–1212. [Google Scholar] [CrossRef] [Green Version]
- Marcus, Y.; Smith, A.L.; Korobov, M.V.; Mirakyan, A.L.; Avramenko, N.V.; Stukalin, E.B. Solubility of C60 Fullerene. J. Phys. Chem. B 2001, 105, 2499–2506. [Google Scholar] [CrossRef]
- Sivaraman, N.; Dhamodaran, R.; Kaliappan, I.; Srinivasan, T.G.; Vasudeva Rao, P.R.P.; Mathews, C.K.C. Solubility of C70in Organic Solvents. Fuller. Sci. Technol. 1994, 2, 233–246. [Google Scholar] [CrossRef]
- Ichida, M.; Sakai, M.; Yajima, T.; Nakamura, A. Luminescence properties of C70 in solutions and solids. J. Lumin. 1997, 72–74, 499–500. [Google Scholar] [CrossRef]
- Orlandi, G.; Negri, F. Electronic states and transitions in C60 and C70 fullerenes. Photochem. Photobiol. Sci. 2002, 1, 289–308. [Google Scholar] [CrossRef]
- Pavlovich, V.S.; Shpilevsky, E.M. Absorption and fluorescence spectra of C60 fullerene concentrated solutions in hexane and polystyrene at 77–300 K. J. Appl. Spectrosc. 2010, 77, 335–342. [Google Scholar] [CrossRef]
- Palewska, K.; Sworakowski, J.; Chojnacki, H.; Meister, E.C.; Wild, U.P. A photoluminescence study of fullerenes: Total luminescence spectroscopy of C60 and C70. J. Phys. Chem. 1993, 97, 12167–12172. [Google Scholar] [CrossRef]
- Wang, Y. Photophysical properties of fullerenes and fullerene/N,N-diethylaniline charge-transfer complexes. J. Phys. Chem. 1992, 96, 764–767. [Google Scholar] [CrossRef]
- Cavar, E.; Blum, M.C.; Pivetta, M.; Patthey, F.; Chergui, M.; Schneider, W.D. Fluorescence and phosphorescence from individual molecules excited by local electron tunneling. Phys. Rev. Lett. 2005, 95, 196102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibley, S.P.; Argentine, S.M.; Francis, A.H. A photoluminescence study of C60 and C70. Chem. Phys. Lett. 1992, 188, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Leach, S.; Vervloet, M.; Desprès, A.; Bréheret, E.; Hare, J.P.; John Dennis, T.; Kroto, H.W.; Taylor, R.; Walton, D.R.M. Electronic spectra and transitions of the fullerene C60. Chem. Phys. 1992, 160, 451–466. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Banerjee, M.; Mukherjee, A.K. Study of the formation equilibria of electron donor-acceptor complexes between [60]fullerene and methylbenzenes by absorption spectrometric method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57, 1463–1470. [Google Scholar] [CrossRef]
- Hiraoka, K.; Nara, M.; Iijima, Y. Luminescence emission and excitation spectra of benzene thin film under slow electron impact at 77 K. J. Phys. Chem. 1983, 87, 3959–3961. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Nayak, S.K.; Chattopadhyay, S.K.; Banerjee, M.; Mukherjee, A.K. Absorption spectroscopic study of EDA complexes of. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57, 309–313. [Google Scholar] [CrossRef]
- Dhawan, A.; Taurozzi, J.S.; Pandey, A.K.; Shan, W.; Miller, S.M.; Hashsham, S.A.; Tarabara, V.V. Stable colloidal dispersions of C60 fullerenes in water: Evidence for genotoxicity. Environ. Sci. Technol. 2006, 40, 7394–7401. [Google Scholar] [CrossRef]
- Talyzin, A.; Jansson, U. C60 and C70 Solvates Studied by Raman Spectroscopy. J. Phys. Chem. B 2000, 104, 5064–5071. [Google Scholar] [CrossRef]
- Rice, J.H.; Galaup, J.-P.; Leach, S. Fluorescence and phosphorescence spectroscopy of C70 in toluene at 5 K: Site dependent low lying excited states. Chem. Phys. 2002, 279, 23–41. [Google Scholar] [CrossRef]
- Qaiser, D.; Khan, M.S.; Singh, R.D.; Khan, Z.H. Time resolved spectroscopy and gain studies of Fullerenes C60 and C70. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 113, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Ruoff, R.S.; Kadish, K.M.; Group, E.S.F. Proceedings of the Symposium on Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials; Electrochemical Society: Philadelphia, PA, USA, 1995. [Google Scholar]
- Çiçek, B.; Kenar, A.; Nazir, H. Simultaneous Determination of C60and C70fullerenes by a Spectrophotometric Method. Fuller. Sci. Technol. 2001, 9, 103–111. [Google Scholar] [CrossRef]
- Dudnik, A.I.; Osipova, I.V.; Nikolaev, N.S.; Churilov, G.N. Comparative analysis of two methods for synthesis of fullerenes at different helium pressures. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 697–701. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Bolotnik, T.A.; Volkov, D.S.; Korobov, M.V.; Proskurnin, M.A. Approaches to the determination of C60 and C70 fullerene and their mixtures in aqueous and organic solutions. Nanosyst. Phys. Chem. Math. 2016, 7, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Vencel, T.; Donovalová, J.; Gáplovský, A.; Kimura, T.; Toma, S. Oxygen Exclusion from the Organic Solvents Using Ultrasound and Comparison with Other Common Techniques Used in Photochemical Experiments. Chem. Pap. 2005, 59, 271–274. [Google Scholar]
- Alargova, R.G.; Deguchi, S.; Tsujii, K. Stable colloidal dispersions of fullerenes in polar organic solvents. J. Am. Chem. Soc. 2001, 123, 10460–10467. [Google Scholar] [CrossRef]









| Fullerene/Solvent | EEM Region | Excitation/Emission Wavelength, nm | LOD (cmin), nM | Regression Equation and Concentration Range (in Parentheses) |
|---|---|---|---|---|
| C70 in benzene | A | 490/660 | 2 | I = (4.1 ± 1.0) × 108 × c + (70 ± 10), n = 4, r = 0.9990 (0.03 ÷ 0.10 μM) |
| I = (5.2 ± 0.8) × 108 × c + (60 ± 10), n = 5, r = 0.9985 (0.10 ÷ 0.35 μM) | ||||
| C60 in n-hexane | B (sum) | 310/740 | 7 | I = (2.0 ± 0.1) × 107 × c + (300 ± 100), n = 7, r = 0.9984 (10.0 ÷ 50.0 μM) |
| C | 310/420 | 1 | I = (1.3 ± 0.1) × 108 × c + (40 ± 10), n = 7, r = 0.9980 (0.03 ÷ 1.00 μM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikheev, I.V.; Verkhovskii, V.A.; Byvsheva, S.M.; Volkov, D.S.; Proskurnin, M.A.; Ivanov, V.K. Simultaneous Quantification of Fullerenes C60 and C70 in Organic Solvents by Excitation–Emission Matrix Fluorescence Spectroscopy. Inorganics 2023, 11, 136. https://doi.org/10.3390/inorganics11040136
Mikheev IV, Verkhovskii VA, Byvsheva SM, Volkov DS, Proskurnin MA, Ivanov VK. Simultaneous Quantification of Fullerenes C60 and C70 in Organic Solvents by Excitation–Emission Matrix Fluorescence Spectroscopy. Inorganics. 2023; 11(4):136. https://doi.org/10.3390/inorganics11040136
Chicago/Turabian StyleMikheev, Ivan V., Viktor A. Verkhovskii, Sofiya M. Byvsheva, Dmitry S. Volkov, Mikhail A. Proskurnin, and Vladimir K. Ivanov. 2023. "Simultaneous Quantification of Fullerenes C60 and C70 in Organic Solvents by Excitation–Emission Matrix Fluorescence Spectroscopy" Inorganics 11, no. 4: 136. https://doi.org/10.3390/inorganics11040136