The Functionalization of PES/SAPO-34 Mixed Matrix Membrane with [emim][Tf2N] Ionic Liquid to Improve CO2/N2 Separation Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. SAPO-34 Synthesis and Characterization
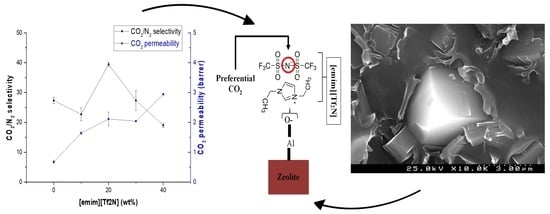
2.2. Gas Permeation Tests
2.3. Characterization of Mixed Matrix PES/SAPO-34 Membranes
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization of SAPO-34
3.3. Synthesis of Mixed Matrix PES/SAPO-34 Membranes
3.4. Gas Permeations Tests
3.5. Characterization of Mixed Matrix PES/SAPO-34 Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robeson, L.M. The Upper Bound Revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Li, S.; Falconer, J.L.; Noble, R.D. Improved SAPO-34 Membranes for CO2/CH4 Separations. Adv. Mater. 2006, 18, 2601–2603. [Google Scholar] [CrossRef]
- Usman, M. Recent Progress of SAPO-34 Zeolite Membranes for CO2 Separation: A Review. Membranes 2022, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M. Correlation of Separation Factor versus Permeability for Polymeric Membranes. J. Memb. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. Polymer Membranes for Gas Separation. Curr. Opin. Solid State Mater. Sci. 1999, 4, 549–552. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Kumakiri, I.; Tanaka, K.; Chen, X.; Kita, H. Preparation and Permeation Properties of PeSU-Based Mixed Matrix Membranes with Nano-Sized CHA Zeolites. J. Chem. Eng. Jpn. 2019, 52, 514–520. [Google Scholar] [CrossRef]
- Cardoso, J.S.; Lin, Z.; Brito, P.; Gando-Ferreira, L.M. Surface Functionalized SAPO-34 for Mixed Matrix Membranes in CO2/CH4 and CO2/N2. Sep. Purif. Rev. 2023, 1–14. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson Upper Bounds for CO2/CH4 and CO2/N2 Separations Using a Series of Ultrapermeable Benzotriptycene-Based Polymers of Intrinsic Microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, M.; Carreon, M.A.; Song, Z.; Zhou, R.; Li, S.; Feng, X.; Zhou, S.J.; Zong, Z.; Meyer, H.S. SAPO-34 Membranes for N2/CH4 Separation: Preparation, Characterization, Separation Performance and Economic Evaluation. J. Memb. Sci. 2015, 487, 141–151. [Google Scholar] [CrossRef]
- Mannan, H.A.; Nasir, R.; Mukhtar, H.; Mohshim, D.F.; Shaharun, M.S. 11—Role of Ionic Liquids in Eliminating Interfacial Defects in Mixed Matrix Membranes. In Interfaces in Particle and Fibre Reinforced Composites; Goh, K.L., Rangika, A.M.K., De Silva, R.T., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 269–309. ISBN 978-0-08-102665-6. [Google Scholar]
- Tan, X.; Robijns, S.; Thür, R.; Ke, Q.; De Witte, N.; Lamaire, A.; Li, Y.; Aslam, I.; Van Havere, D.; Donckels, T.; et al. Truly Combining the Advantages of Polymeric and Zeolite Membranes for Gas Separations. Science 2022, 378, 1189–1194. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Mukhtar, H.; Mohshim, D.F.; Nasir, R.; Man, Z. Effect of Different Organic Amino Cations on SAPO-34 for PES/SAPO-34 Mixed Matrix Membranes toward CO2/CH4 Separation. J. Appl. Polym. Sci. 2016, 133, 43387. [Google Scholar] [CrossRef]
- Liu, Y.; Takata, K.; Kita, H.; Tanaka, K. Investigation of Gas Diffusivity and Solubility of PES Based Mixed Matrix Membranes Using Commercial SAPO-34 Zeolite. Trans. Mat. Res. Soc. Jpn. 2021, 46, 39–43. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Leo, C.P.; Mohammad, A.W.; Shaari, N.; Ang, W.L. Recent Progress in the Development of Ionic Liquid-Based Mixed Matrix Membrane for CO2 Separation: A Review. Int. J. Energy Res. 2021, 45, 9800–9830. [Google Scholar]
- Oral, E.E.; Yilmaz, L.; Kalipcilar, H. Effect of Gas Permeation Temperature and Annealing Procedure on the Performance of Binary and Ternary Mixed Matrix Membranes of Polyethersulfone, SAPO-34, and 2-Hydroxy 5-Methyl Aniline. J. Appl. Polym. Sci. 2014, 131, 8498–8505. [Google Scholar] [CrossRef]
- Ismail, A.F.; Kusworo, T.D.; Mustafa, A. Enhanced Gas Permeation Performance of Polyethersulfone Mixed Matrix Hollow Fiber Membranes Using Novel Dynasylan Ameo Silane Agent. J. Memb. Sci. 2008, 319, 306–312. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Khoo, C.P.; Leo, C.P.; Ahmad, A.L. The Effects of Solvents on the Modification of SAPO-34 Zeolite Using 3-Aminopropyl Trimethoxy Silane for the Preparation of Asymmetric Polysulfone Mixed Matrix Membrane in the Application of CO2 Separation. Microporous Mesoporous Mater. 2014, 192, 52–59. [Google Scholar] [CrossRef]
- Suer, M.G.; Baç, N.; Yilmaz, L. Gas Permeation Characteristics of Polymer-Zeolite Mixed Matrix Membranes. J. Memb. Sci. 1994, 91, 77–86. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Amino-Functionalized SAPO-34 Membranes for CO2/CH4 and CO2/N2 Separation. Langmuir 2011, 27, 2888–2894. [Google Scholar] [CrossRef]
- Lixiong, Z.; Mengdong, J.; Enze, M. Synthesis of SAPO-34/Ceramic Composite Membranes. Stud. Surf. Sci. Catal. 1997, 105, 2211–2216. [Google Scholar] [CrossRef]
- Makertihartha, I.G.B.N.; Kencana, K.S.; Dwiputra, T.R.; Khoiruddin, K.; Mukti, R.R.; Wenten, I.G. Silica Supported SAPO-34 Membranes for CO2/N2 Separation. Microporous Mesoporous Mater. 2020, 298, 110068. [Google Scholar] [CrossRef]
- Cakal, U.; Yilmaz, L.; Kalipcilar, H. Effect of Feed Gas Composition on the Separation of CO2/CH4 Mixtures by PES-SAPO 34-HMA Mixed Matrix Membranes. J. Memb. Sci. 2012, 417–418, 45–51. [Google Scholar] [CrossRef]
- Mohshim, D.F.; Mukhtar, H.; Man, Z. The Effect of Incorporating Ionic Liquid into Polyethersulfone-SAPO34 Based Mixed Matrix Membrane on CO2 Gas Separation Performance. Sep. Purif. Technol. 2014, 135, 252–258. [Google Scholar] [CrossRef]
- Nasir, R.; Ahmad, N.N.R.; Mukhtar, H.; Mohshim, D.F. Effect of Ionic Liquid Inclusion and Amino-Functionalized SAPO-34 on the Performance of Mixed Matrix Membranes for CO2/CH4 separation. J. Environ. Chem. Eng. 2018, 6, 2363–2368. [Google Scholar] [CrossRef]
- Mannan, H.A.; Mukhtar, H.; Shahrun, M.S.; Bustam, M.A.; Man, Z.; Bakar, M.Z.A. Effect of [EMIM][Tf2N] Ionic Liquid on Ionic Liquid-Polymeric Membrane (ILPM) for CO2/CH4 Separation. Procedia Eng. 2016, 148, 25–29. [Google Scholar] [CrossRef]
- Ding, S.S.; Li, X.; Ding, S.S.; Zhang, W.; Guo, R.; Zhang, J. Ionic Liquid-Decorated Nanocages for Cooperative CO2 Transport in Mixed Matrix Membranes. Sep. Purif. Technol. 2020, 239, 116539. [Google Scholar] [CrossRef]
- Cadena, C.; Anthony, J.L.; Shah, J.K.; Morrow, T.I.; Brennecke, J.F.; Maginn, E.J. Why Is CO2 so Soluble in Imidazolium-Based Ionic Liquids? J. Am. Chem. Soc. 2004, 126, 5300–5308. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Dong, H.; Zhao, Z.; Zhang, S.; Huang, Y. Carbon Capture with Ionic Liquids: Overview and Progress. Energy Environ. Sci. 2012, 5, 6668–6681. [Google Scholar] [CrossRef]
- Li, S.; Falconer, J.L.; Noble, R.D. SAPO-34 Membranes for CO2/CH4 Separations: Effect of Si/Al Ratio. Microporous Mesoporous Mater. 2008, 110, 310–317. [Google Scholar] [CrossRef]
- Yang, H.; Liu, X.; Lu, G.; Wang, Y. Synthesis of SAPO-34 Nanoplates via Hydrothermal Method. Microporous Mesoporous Mater. 2016, 225, 144–153. [Google Scholar] [CrossRef]
- Li, Z.; Martínez-Triguero, J.; Yu, J.; Corma, A. Conversion of Methanol to Olefins: Stabilization of Nanosized SAPO-34 by Hydrothermal Treatment. J. Catal. 2015, 329, 379–388. [Google Scholar] [CrossRef]
- Sena, F.C.; De Souza, B.F.; De Almeida, N.C.; Cardoso, J.S.; Fernandes, L.D. Influence of Framework Composition over SAPO-34 and MeAPSO-34 Acidity. Appl. Catal. A Gen. 2011, 406, 59–62. [Google Scholar] [CrossRef]
- Maeda, Y.; Paul, D.R. Effect of Antiplasticization on Selectivity and Productivity of Gas Separation Membranes. J. Memb. Sci. 1987, 30, 1–9. [Google Scholar] [CrossRef]
- Mahajan, R.; Burns, R.; Schaeffer, M.; Koros, W.J. Challenges in Forming Successful Mixed Matrix Membranes with Rigid Polymeric Materials. J. Appl. Polym. Sci. 2002, 86, 881–890. [Google Scholar] [CrossRef]
- Şen, D.; Kalipçilar, H.; Yilmaz, L. Development of Polycarbonate Based Zeolite 4A Filled Mixed Matrix Gas Separation Membranes. J. Memb. Sci. 2007, 303, 194–203. [Google Scholar] [CrossRef]
- Naderi, A.; Yong, W.F.; Xiao, Y.; Chung, T.S.; Weber, M.; Maletzko, C. Effects of Chemical Structure on Gas Transport Properties of Polyethersulfone Polymers. Polymer 2018, 135, 76–84. [Google Scholar] [CrossRef]
- Liu, S.H.; Chen, C.C.; Zhang, B.; Wu, J.H. Fire and Explosion Hazards of 1-Ethyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide. RSC Adv. 2020, 10, 22468–22479. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Forsyth, S.A.; Pringle, J.M.; MacFarlane, D.R. Ionic Liquids—An Overview. Aust. J. Chem. 2004, 57, 113–119. [Google Scholar] [CrossRef]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion Effects on Gas Solubility in Ionic Liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef]
- Noel Jacob, K.; Senthil Kumar, S.; Thanigaivelan, A.; Tarun, M.; Mohan, D. Sulfonated Polyethersulfone-Based Membranes for Metal Ion Removal via a Hybrid Process. J. Mater. Sci. 2014, 49, 114–122. [Google Scholar] [CrossRef]
- Alenazi, N.; Hussein, M.; Alamry, K.; Asiri, A. Nanocomposite-Based Aminated Polyethersulfone and Carboxylate Activated Carbon for Environmental Application. A Real Sample Analysis. C 2018, 4, 30. [Google Scholar] [CrossRef]
- Ran, F. Polyethersulfone Membrane. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–2. [Google Scholar]
- Cardoso, J.S.; Fonseca, J.P.; Lin, Z.; Brito, P.; Gando-Ferreira, L.M. Optimization and Performance Studies of PES/SAPO-34 Membranes for CO2/N2 Gas Separation. Microporous Mesoporous Mater. 2023, 364, 112845. [Google Scholar] [CrossRef]














| Filler | %Filler | Polymer | PCO2 | αCO2/CH4 | αCO2/N2 | Reference |
|---|---|---|---|---|---|---|
| Neat | - | PES | 2.6 barrer | - | 18.57 | [18] |
| Neat SAPO-34 (stainless steel) | - | - | 328 GPU | 159 | 29 | [19] |
| Neat SAPO-34 (porous alumina) | - | - | 892 GPU | - | 7.09 | [20] |
| Neat SAPO-34 (silica) | - | - | 6000 GPU | - | 53 | [21] |
| Neat | - | PES | 6.7 barrer | 37.8 | 34.04 | [6] |
| SAPO-34 | 20 wt% | PES | 8.25 barrer | 42.6 | 34.51 | |
| SAPO-34 | 30 wt% | PES | 8.9 barrer | 48.3 | 7.08 | |
| Neat | - | PES | 4.45 barrer | 33.2 | - | [22] |
| HMA 10% | - | PES | 0.8 barrer | 32.3 | - | |
| SAPO-34 | 20 wt% | PES | 5.7 barrer | 37 | - | |
| SAPO-34 + HMA 10% | 20 wt% | PES | 1.3 barrer | 44.7 | - | |
| HMA 4% | - | PES | 5.1 barrer | 39.3 | - | [15] |
| SAPO-34 | 20 wt% | PES | 13.8 barrer | 32.7 | - | |
| SAPO-34 + HMA 4% | 20 wt% | PES | 7.8 barrer | 41.6 | - | |
| SAPO-34 | 20 wt% | PES | 85.69 GPU | 20.67 | - | [23] |
| SAPO-34 + IL 5% | 20 wt% | PES | 230.81 GPU | 46.20 | - | |
| SAPO-34 + IL 10% | 20 wt% | PES | 255.69 GPU | 58.83 | - | |
| SAPO-34 + IL 15% | 20 wt% | PES | 279.26 GPU | 60.62 | - | |
| SAPO-34 + IL 20% | 20 wt% | PES | 300 GPU | 62.58 | - | |
| SAPO-34 | 20 wt% | PES | 30 GPU | 1.3 | - | [12] |
| SAPO-34 + m-EDA | 20 wt% | PES | 10 GPU | 12.14 | - | |
| SAPO-34 | 20 wt% | PES | 50 GPU | 2.5 | - | [24] |
| SAPO-34 + IL | 20 wt% | PES | 0.03 GPU | 4.9 | - | |
| SAPO-34 + m-EDA + IL | 20 wt% | PES | 0.09 GPU | 26.5 | - | |
| SAPO-34 + m-HA + IL | 20 wt% | PES | 0.045 GPU | 37.2 | - |
| Powder | Si/Al Molar Ratio | |
|---|---|---|
| Gel | Crystal | |
| SAPO-34 sample | 0.30 | 0.29 |
| Sample | SBET (m2 g−1) | SLangmuir (m2 g−1) | Sext (m2 g−1) | Smic (m2 g−1) | Vmic (mm3 g−1) |
|---|---|---|---|---|---|
| SAPO-34 | 464 | 707 | 9 | 455 | 0.249 |
| Sample | αCO2/N2 | PCO2 (barrer) | PN2 (barrer) |
|---|---|---|---|
| Neat PES | 21.35 ± 3.62 | 1.04 ± 0.003 | 0.05 ± 0.0090 |
| PES/SAPO-34 | 27.31 ± 1.08 | 0.67 ± 0.04 | 0.025 ± 0.0005 |
| PES/SAPO-34/[emim][Tf2N]10 | 22.71 ± 2.14 | 1.64 ± 0.05 | 0.072 ± 0.009 |
| PES/SAPO-34/[emim][Tf2N]20 | 39.44 ± 0.75 | 2.11 ± 0.23 | 0.054 ± 0.007 |
| PES/SAPO-34/[emim][Tf2N]30 | 27.36 ± 3.28 | 2.04 ± 0.01 | 0.075 ± 0.020 |
| PES/SAPO-34/[emim][Tf2N]40 | 19.00 ± 0.70 | 2.94 ± 0.03 | 0.155 ± 0.007 |
| Sample | Average Contact Angle (°) |
|---|---|
| PES | 70 ± 2 |
| PES/SAPO-34 | 71 ± 2 |
| PES/SAPO-34/[emim][Tf2N]20 | 61 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, J.S.; Lin, Z.; Brito, P.; Gando-Ferreira, L.M. The Functionalization of PES/SAPO-34 Mixed Matrix Membrane with [emim][Tf2N] Ionic Liquid to Improve CO2/N2 Separation Properties. Inorganics 2023, 11, 447. https://doi.org/10.3390/inorganics11110447
Cardoso JS, Lin Z, Brito P, Gando-Ferreira LM. The Functionalization of PES/SAPO-34 Mixed Matrix Membrane with [emim][Tf2N] Ionic Liquid to Improve CO2/N2 Separation Properties. Inorganics. 2023; 11(11):447. https://doi.org/10.3390/inorganics11110447
Chicago/Turabian StyleCardoso, Jonathan S., Zhi Lin, Paulo Brito, and Licínio M. Gando-Ferreira. 2023. "The Functionalization of PES/SAPO-34 Mixed Matrix Membrane with [emim][Tf2N] Ionic Liquid to Improve CO2/N2 Separation Properties" Inorganics 11, no. 11: 447. https://doi.org/10.3390/inorganics11110447