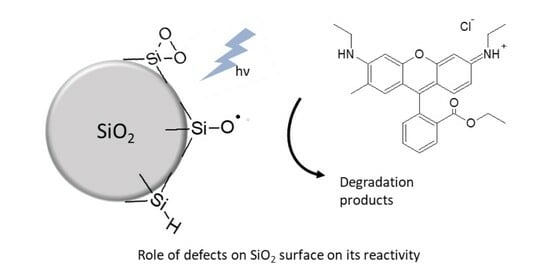
Role of Surface Defects on Photoinduced Reactivity in SiO2 Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Morphological Characterization of the Materials
2.1.1. TEM Analyses
2.1.2. Surface Analyses
2.1.3. Zeta Potential Measurements
2.1.4. Spectral Characterization
2.1.5. Photodegradation of R6G in Ethanol and Aqueous Solutions
2.1.6. Degradation Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Nanoparticles
4.2.1. Preparation of Silica Nanoparticles
4.2.2. Preparation of Amine- and Phosphonate-Functionalized Silica Nanoparticles
4.3. Nanoparticles Characterization
4.4. Zeta Potential Measurment
4.5. UV-Vis Absorption and Fluorescence Spectra of Nanoparticles Suspensions and Dye Solutions
4.6. Photodegradation Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arcaro, S.; Wermuth, T.B.; Zampiva, R.Y.S.; Venturini, J.; Ten Caten, C.S.; Bergmann, C.P.; Alves, A.K.; De Oliveira, A.P.N.; Moreno, R. Li2O-ZrO2-SiO2/Al2O3 Nanostructured Composites for Microelectronics Applications. J. Eur. Ceram. Soc. 2019, 39, 491–498. [Google Scholar] [CrossRef]
- Ayoub, B.; Lhostis, S.; Moreau, S.; Mattei, J.-G.; Mukhtarov, A.; Frémont, H. Investigation into Cu Diffusion at the Cu/SiO2 Hybrid Bonding Interface of 3D Stacked Integrated Circuits. Microelectron. Reliab. 2023, 143, 114934. [Google Scholar] [CrossRef]
- Raffaini, G.; Catauro, M. Surface Interactions between Ketoprofen and Silica-Based Biomaterials as Drug Delivery System Synthesized via Sol–Gel: A Molecular Dynamics Study. Materials 2022, 15, 2759. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, Y.; Shahid, H.; Rashid, M.A.; Alhamhoom, Y.; Kazi, M. Developing of SiO2 Nanoshells Loaded with Fluticasone Propionate for Potential Nasal Drug Delivery: Determination of Pro-Inflammatory Cytokines through mRNA Expression. J. Funct. Biomater. 2022, 13, 229. [Google Scholar] [CrossRef]
- Collantes, C.; González Pedro, V.; Bañuls, M.-J.; Maquieira, Á. Monodispersed CsPb2 Br5 @SiO2 Core–Shell Nanoparticles as Luminescent Labels for Biosensing. ACS Appl. Nano Mater. 2021, 4, 2011–2018. [Google Scholar] [CrossRef]
- Selvaggi, R.; Tarpani, L.; Santuari, A.; Giovagnoli, S.; Latterini, L. Silica Nanoparticles Assisted Photodegradation of Acridine Orange in Aqueous Suspensions. Appl. Catal. B Environ. 2015, 168–169, 363–369. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Garud, H.B.; Patil, A.H.; Patil, G.D.; Patil, C.R.; Dongale, T.D.; Patil, P.S. Recent Advancements in Silica Nanoparticles Based Technologies for Removal of Dyes from Water. Colloid Interface Sci. Commun. 2019, 30, 100181. [Google Scholar] [CrossRef]
- El-Shamy, A.G. Novel In-Situ Synthesis of Nano-Silica (SiO2) Embedded into Polyvinyl Alcohol for Dye Removal: Adsorption and Photo-Degradation under Visible Light. Polymer 2022, 242, 124579. [Google Scholar] [CrossRef]
- Quaglia, G.; Ambrogi, V.; Pietrella, D.; Nocchetti, M.; Latterini, L. Solid State Photoreduction of Silver on Mesoporous Silica to Enhance Antifungal Activity. Nanomaterials 2021, 11, 2340. [Google Scholar] [CrossRef]
- Romolini, G.; Gambucci, M.; Ricciarelli, D.; Tarpani, L.; Zampini, G.; Latterini, L. Photocatalytic Activity of Silica and Silica-Silver Nanocolloids Based on Photo-Induced Formation of Reactive Oxygen Species. Photochem. Photobiol. Sci. 2021, 20, 1161–1172. [Google Scholar] [CrossRef]
- Latterini, L.; Tarpani, L. Hierarchical Assembly of Nanostructures to Decouple Fluorescence and Photothermal Effect. J. Phys. Chem. C 2011, 115, 21098–21104. [Google Scholar] [CrossRef]
- Gubala, V.; Giovannini, G.; Kunc, F.; Monopoli, M.P.; Moore, C.J. Dye-Doped Silica Nanoparticles: Synthesis, Surface Chemistry and Bioapplications. Cancer Nanotechnol. 2020, 11, 1. [Google Scholar] [CrossRef]
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Tarpani, L.; Ruhlandt, D.; Latterini, L.; Haehnel, D.; Gregor, I.; Enderlein, J.; Chizhik, A.I. Photoactivation of Luminescent Centers in Single SiO2 Nanoparticles. Nano Lett. 2016, 16, 4312–4316. [Google Scholar] [CrossRef]
- Gogoi, S.H.; Banerjee, A. Datta, Photoluminescent Silica Nanostructures and Nanohybrids. ChemPhysChem 2022, 23, e202200280. [Google Scholar] [CrossRef]
- Glinka, Y.D.; Lin, S.-H.; Chen, Y.-T. Time-Resolved Photoluminescence Study of Silica Nanoparticles as Compared to Bulk Type-III Fused Silica. Phys. Rev. B 2002, 66, 035404. [Google Scholar] [CrossRef]
- Ogata, A.; Kazusaka, A.; Enyo, M. Photoactivation of Silica Gel with UV Light during the Reaction of Carbon Monoxide with Oxygen. J. Phys. Chem. 1986, 90, 5201–5205. [Google Scholar] [CrossRef]
- Yoshida, H.; Kimura, K.; Inaki, Y.; Hattori, T. Catalytic Activity of FSM-16 for Photometathesis of Propene. Chem. Commun. 1997, 1, 129–130. [Google Scholar] [CrossRef]
- Yoshida, H.; Tanaka, T.; Matsuo, S.; Funabiki, T.; Yoshida, S. Alkene Metathesis on Photoirradiated Silica Surface. J. Chem. Soc. Chem. Commun. 1995, 7, 761–762. [Google Scholar] [CrossRef]
- Inaki, Y.; Yoshida, H.; Kimura, K.; Inagaki, S.; Fukushima, Y.; Hattori, T. Photometathesis Activity and Thermal Stability of Two Types of Mesoporous Silica Materials, FSM-16 and MCM-41. Phys. Chem. Chem. Phys. 2000, 2, 5293–5297. [Google Scholar] [CrossRef]
- Inaki, Y.; Yoshida, H.; Yoshida, T.; Hattori, T. Active Sites on Mesoporous and Amorphous Silica Materials and Their Photocatalytic Activity: An Investigation by FTIR, ESR, VUV−UV and Photoluminescence Spectroscopies. J. Phys. Chem. B 2002, 106, 9098–9106. [Google Scholar] [CrossRef]
- Spallino, L.; Vaccaro, L.; Agnello, S.; Cannas, M. Effects Induced by UV Laser Radiation on the Blue Luminescence of Silica Nanoparticles. J. Lumin. 2013, 138, 39–43. [Google Scholar] [CrossRef]
- Skuja, L. Optically Active Oxygen-Deficiency-Related Centers in Amorphous Silicon Dioxide. J. Non-Cryst. Solids 1998, 239, 16–48. [Google Scholar] [CrossRef]
- Rahman, I.A.; Vejayakumaran, P.; Sipaut, C.S.; Ismail, J.; Chee, C.K. Size-Dependent Physicochemical and Optical Properties of Silica Nanoparticles. Mater. Chem. Phys. 2009, 114, 328–332. [Google Scholar] [CrossRef]
- Bergamini, R.B.M.; Azevedo, E.B.; de Araújo, L.R.R. Heterogeneous Photocatalytic Degradation of Reactive Dyes in Aqueous TiO2 Suspensions: Decolorization Kinetics. Chem. Eng. J. 2009, 149, 215–220. [Google Scholar] [CrossRef]
- Flomenbom, O.; Velonia, K.; Loos, D.; Masuo, S.; Cotlet, M.; Engelborghs, Y.; Hofkens, J.; Rowan, A.E.; Nolte, R.J.M.; Van der Auweraer, M.; et al. Stretched Exponential Decay and Correlations in the Catalytic Activity of Fluctuating Single Lipase Molecules. Proc. Natl. Acad. Sci. USA 2005, 102, 2368–2372. [Google Scholar] [CrossRef]
- Sulpizi, M.; Gaigeot, M.-P.; Sprik, M. The Silica–Water Interface: How the Silanols Determine the Surface Acidity and Modulate the Water Properties. J. Chem. Theory Comput. 2012, 8, 1037–1047. [Google Scholar] [CrossRef]
- Wu, D.; Guo, X.; Sun, H.; Navrotsky, A. Energy Landscape of Water and Ethanol on Silica Surfaces. J. Phys. Chem. C 2015, 119, 15428–15433. [Google Scholar] [CrossRef]
- Morris, C.A.; Rolison, D.R.; Swider-Lyons, K.E.; Osburn-Atkinson, E.J.; Merzbacher, C.I. Modifying Nanoscale Silica with Itself: A Method to Control Surface Properties of Silica Aerogels Independently of Bulk Structure. J. Non-Cryst. Solids 2001, 285, 29–36. [Google Scholar] [CrossRef]
- Santra, S.; Zhang, P.; Wang, K.; Tapec, R.; Tan, W. Conjugation of Biomolecules with Luminophore-Doped Silica Nanoparticles for Photostable Biomarkers. Anal. Chem. 2001, 73, 4988–4993. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]






| Catalyst | Surface Area (m2/g) | Z (mV) in EtOH | Z (mV) in H2O |
|---|---|---|---|
| SNP | 102.4 | −20 ± 5 | −37 ± 4 |
| SNP-APTES | 72.9 | +5 ± 5 | −4 ± 3 |
| SNP–phosphonate | 73.1 | −30 ± 5 | −56 ± 5 |
| Material | Band Gap (eV) |
|---|---|
| SNP | 5.08 |
| SNP–APTES | 5.05 |
| SNP–phosphonate | 4.90 |
| Catalyst | Photodegradation Efficiency (R%) | t1/2 (min) |
|---|---|---|
| SNP | 76 | 5.7 |
| SNP–APTES | 43 | 243 |
| SNP–phosphonate | 13 | 3586 |
| Defect Type | SNP | SNP–APTES | SNP–Phosphonate |
|---|---|---|---|
| ≡Si–H | ● | ● | ● |
| ≡Si–Si≡ | ● | ● | ● |
| ≡Si–O● | ● | ● | ● |
| O divacancy | ● | ||
| O excess | ● | ● |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amato, R.; Quaglia, G.; Selvaggi, R.; Marmottini, F.; Latterini, L. Role of Surface Defects on Photoinduced Reactivity in SiO2 Nanoparticles. Inorganics 2023, 11, 430. https://doi.org/10.3390/inorganics11110430
D’Amato R, Quaglia G, Selvaggi R, Marmottini F, Latterini L. Role of Surface Defects on Photoinduced Reactivity in SiO2 Nanoparticles. Inorganics. 2023; 11(11):430. https://doi.org/10.3390/inorganics11110430
Chicago/Turabian StyleD’Amato, Roberto, Giulia Quaglia, Roberta Selvaggi, Fabio Marmottini, and Loredana Latterini. 2023. "Role of Surface Defects on Photoinduced Reactivity in SiO2 Nanoparticles" Inorganics 11, no. 11: 430. https://doi.org/10.3390/inorganics11110430