Facile Construction of Bi2Sn2O7/g-C3N4 Heterojunction with Enhanced Photocatalytic Degradation of Norfloxacin
Abstract
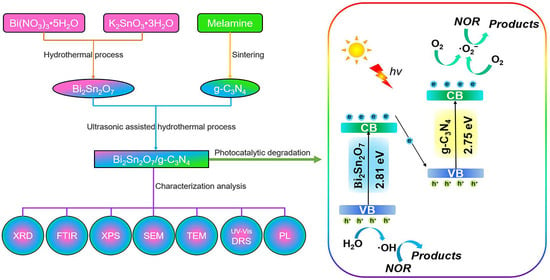
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. FTIR Analysis
2.3. XPS Analysis
2.4. SEM and TEM Analysis
2.5. UV–Vis DRS Analysis
2.6. PL Analysis
2.7. Photocatalytic Performance

| Sample Name | Degradation (%) | K (min−1) | R2 |
|---|---|---|---|
| BSO | 40% | 0.00325 | 0.95248 |
| CN | 31% | 0.00216 | 0.9814 |
| 10BSCN | 78% | 0.00703 | 0.98684 |
| 20BSCN | 94% | 0.01261 | 0.96041 |
| 30BSCN | 70% | 0.0068 | 0.98331 |
| 40BSCN | 87% | 0.00997 | 0.97395 |
| 50BSCN | 85% | 0.00932 | 0.98105 |
| Sample Name | Degradation | Time | Light Source | Target | Reference |
|---|---|---|---|---|---|
| CeO2/g-C3N4 | 88.6% | 60 min | Visible light | Norfloxacin | [41] |
| NiWO4/g-C3N4 | 97% | 60 min | Visible light | Norfloxacin | [42] |
| LaNiO3/g-C3N4 | 96% | 300 min | Visible light | Tetracycline | [43] |
| TiO2/g-C3N4 | 99.4% | 120 min | Visible light | Tetracycline | [44] |
| ZnIn2S4/g-C3N4 | 98% | 300 min | Visible light | Metronidazole | [45] |
| BiOCl/g-C3N4 | 95% | 180 min | Visible light | Metronidazole | [46] |
| Bi2Sn2O7/g-C3N4 | 94% | 180 min | Visible light | Norfloxacin | This work |
2.8. Possible Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of g-C3N4 Photocatalyst
3.3. Synthesis of Bi2Sn2O7 Photocatalyst
3.4. Fabrication of Bi2Sn2O7/g-C3N4 Heterojunction Photocatalysts
3.5. Characterization
3.6. Photocatalytic Activity and Stability Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Li, Y.; Zhang, G.; Li, J.; Wu, X. 0D Bi Nanodots/2D Bi3NbO7 Nanosheets Heterojunctions for Efficient Visible Light Photocatalytic Degradation of Antibiotics: Enhanced Molecular Oxygen Activation and Mechanism Insight. Appl. Catal. B Environ. 2019, 240, 39–49. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Xin, Y.; Chai, C.; Chen, Q. Construction of Immobilized CuS/TiO2 Nanobelts Heterojunction Photocatalyst for Photocatalytic Degradation of Enrofloxacin: Synthesis, Characterization, Influencing Factors and Mechanism Insight. J. Chem. Technol. Biotechnol. 2019, 94, 2219–2228. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Ha, E.; Zhang, H.; Li, C. Reduced Graphene Oxide/Bi4O5Br2 Nanocomposite with Synergetic Effects on Improving Adsorption and Photocatalytic Activity for the Degradation of Antibiotics. Chemosphere 2021, 265, 129013. [Google Scholar] [CrossRef]
- Kumar, R.; Barakat, M.A.; Al-Mur, B.A.; Alseroury, F.A.; Eniola, J.O. Photocatalytic Degradation of Cefoxitin Sodium Antibiotic Using Novel BN/CdAl2O4 Composite. J. Clean. Prod. 2020, 246, 119076. [Google Scholar] [CrossRef]
- Kamranifar, M.; Allahresani, A.; Naghizadeh, A. Synthesis and Characterizations of a Novel CoFe2O4@CuS Magnetic Nanocomposite and Investigation of Its Efficiency for Photocatalytic Degradation of Penicillin G Antibiotic in Simulated Wastewater. J. Hazard. Mater. 2019, 366, 545–555. [Google Scholar] [CrossRef]
- Li, J.; Fang, W.; Yu, C.; Zhou, W.; Zhu, L.; Xie, Y. Ag-Based Semiconductor Photocatalysts in Environmental Purification. Appl. Surf. Sci. 2015, 358, 46–56. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Khan, S.; Takagi, K.; Suzuki, N.; Teshima, K.; Terashima, C.; Fujishima, A. Photocatalytic Degradation of Bisphenol A Using Titanium Dioxide@nanodiamond Composites under UV Light Illumination. J. Colloid Interface Sci. 2021, 582, 1058–1066. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Li, J.; Wang, K. Novel Three-Dimensional Flowerlike BiOBr/Bi2SiO5 p-n Heterostructured Nanocomposite for Degradation of Tetracycline: Enhanced Visible Light Photocatalytic Activity and Mechanism. ACS Sustain. Chem. Eng. 2018, 6, 14221–14229. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Kong, X.; He, F.; Zhao, R.; Wu, R.; Wei, T.; Wang, L.; Feng, J. A Novel P-N Heterojunction with Staggered Energy Level Based on ZnFe2O4 Decorating SnS2 Nanosheet for Efficient Photocatalytic Degradation. Appl. Surf. Sci. 2020, 510, 145442. [Google Scholar] [CrossRef]
- Wan, H.; Yao, W.; Zhu, W.; Tang, Y.; Ge, H.; Shi, X.; Duan, T. Fe-N Co-Doped SiO2 @TiO2 Yolk-Shell Hollow Nanospheres with Enhanced Visible Light Photocatalytic Degradation. Appl. Surf. Sci. 2018, 444, 355–363. [Google Scholar] [CrossRef]
- Santos, L.M.M.; Nascimento, M.M.; dos Santos Borges, S.; Bomfim, E.; de Jesus Macedo, V.; Silva, L.A. Green Photocatalytic Remediation of Fenthion Using Composites with Natural Red Clay and Non-Toxic Metal Oxides with Visible Light Irradiation. Environ. Technol. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Jalalah, M.; Faisal, M.; Bouzid, H.; Park, J.G.; Al-Sayari, S.A.; Ismail, A.A. Comparative Study on Photocatalytic Performances of Crystalline α- and β-Bi2O3 Nanoparticles under Visible Light. J. Ind. Eng. Chem. 2015, 30, 183–189. [Google Scholar] [CrossRef]
- Liang, Q.; Jin, J.; Liu, C.; Xu, S.; Li, Z. Constructing a Novel P-n Heterojunction Photocatalyst LaFeO3/g-C3N4 with Enhanced Visible-Light-Driven Photocatalytic Activity. J. Alloys Compd. 2017, 709, 542–548. [Google Scholar] [CrossRef]
- Huang, Y.; Kang, S.; Yang, Y.; Qin, H.; Ni, Z.; Yang, S.; Li, X. Facile Synthesis of Bi/Bi2WO6 Nanocomposite with Enhanced Photocatalytic Activity under Visible Light. Appl. Catal. B Environ. 2016, 196, 89–99. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, Y.; Huang, H.; Zhou, P.; Li, J.; Zhang, L.; Dai, B.; Xu, J.; Zhu, F.; Sheng, N.; et al. A Novel Z-Scheme Ag3VO4/BiVO4 Heterojunction Photocatalyst: Study on the Excellent Photocatalytic Performance and Photocatalytic Mechanism. Appl. Catal. B Environ. 2019, 245, 448–458. [Google Scholar] [CrossRef]
- Song, T.; Yu, X.; Tian, N.; Huang, H. wei Preparation, Structure and Application of g-C3N4/BiOX Composite Photocatalyst. Int. J. Hydrogen Energy 2021, 46, 1857–1878. [Google Scholar] [CrossRef]
- Han, C.; Su, P.; Tan, B.; Ma, X.; Lv, H.; Huang, C.; Wang, P.; Tong, Z.; Li, G.; Huang, Y.; et al. Defective Ultra-Thin Two-Dimensional g-C3N4 Photocatalyst for Enhanced Photocatalytic H2 Evolution Activity. J. Colloid Interface Sci. 2021, 581, 159–166. [Google Scholar] [CrossRef]
- Feng, D.; Cheng, Y.; He, J.; Zheng, L.; Shao, D.; Wang, W.; Wang, W.; Lu, F.; Dong, H.; Liu, H.; et al. Enhanced Photocatalytic Activities of g-C3N4 with Large Specific Surface Area via a Facile One-Step Synthesis Process. Carbon 2017, 125, 454–463. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, X.; Li, R.; Xie, Y.; Tang, Q.; Ren, B. Hydrothermal Synthesis of 2D/2D BiOCl/g-C3N4 Z-Scheme: For TC Degradation and Antimicrobial Activity Evaluation. Opt. Mater. 2020, 108, 110170. [Google Scholar] [CrossRef]
- Hu, K.; Li, R.; Ye, C.; Wang, A.; Wei, W.; Hu, D.; Qiu, R.; Yan, K. Facile Synthesis of Z-Scheme Composite of TiO2 Nanorod/g-C3N4 Nanosheet Efficient for Photocatalytic Degradation of Ciprofloxacin. J. Clean. Prod. 2020, 253, 120055. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, J.; Ji, H.; Li, S.; Chen, L.; Huang, T.; Xu, C.; Chen, X.; Liu, W. Photocatalytic Degradation of Ofloxacin by Perovskite-Type NaNbO3 Nanorods Modified g-C3N4 Heterojunction under Simulated Solar Light: Theoretical Calculation, Ofloxacin Degradation Pathways and Toxicity Evolution. Chem. Eng. J. 2020, 400, 125918. [Google Scholar] [CrossRef]
- Xu, W.; Fang, J.; Chen, Y.; Lu, S.; Zhou, G.; Zhu, X.; Fang, Z. Novel Heterostructured Bi2S3/Bi2Sn2O7 with Highly Visible Light Photocatalytic Activity for the Removal of Rhodamine B. Mater. Chem. Phys. 2015, 154, 30–37. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Hao, J.; Zhang, T.; Sun, Q.; Wang, Y. Boosted Charge Transfer in Dual Z-Scheme BiVO4@ZnIn2S4/Bi2Sn2O7 Heterojunctions: Towards Superior Photocatalytic Properties for Organic Pollutant Degradation. Chemosphere 2021, 276, 130226. [Google Scholar] [CrossRef] [PubMed]
- Elhaddad, E.; Rehman, W.; Waseem, M.; Nawaz, M.; Haq, S.; Guo, C.Y. Fabrication of Highly Efficient Bi2Sn2O7/C3N4 Composite with Enhanced Photocatalytic Activity for Degradation of Organic Pollutants. J. Inorg. Organomet. Polym. Mater. 2021, 31, 172–179. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, J.; Cui, H.; Wang, T. Preparation of Direct Z-Scheme Bi2Sn2O7/g-C3N4 Composite with Enhanced Photocatalytic Performance. J. Photochem. Photobiol. A Chem. 2017, 335, 130–139. [Google Scholar] [CrossRef]
- Heidari, S.; Haghighi, M.; Shabani, M. Ultrasound Assisted Dispersion of Bi2Sn2O7-C3N4 Nanophotocatalyst over Various Amount of Zeolite Y for Enhanced Solar-Light Photocatalytic Degradation of Tetracycline in Aqueous Solution. Ultrason. Sonochem. 2018, 43, 61–72. [Google Scholar] [CrossRef]
- Hu, T.; Dai, K.; Zhang, J.; Zhu, G.; Liang, C. One-Pot Synthesis of Step-Scheme Bi2S3/Porous g-C3N4 Heterostructure for Enhanced Photocatalytic Performance. Mater. Lett. 2019, 257, 126740. [Google Scholar] [CrossRef]
- Wu, J.; Huang, F.; Lü, X.; Chen, P.; Wan, D.; Xu, F. Improved Visible-Light Photocatalysis of Nano-Bi2Sn2O7 with Dispersed s-Bands. J. Mater. Chem. 2011, 21, 3872–3876. [Google Scholar] [CrossRef]
- Miao, X.; Shen, X.; Wu, J.; Ji, Z.; Wang, J.; Kong, L.; Liu, M.; Song, C. Fabrication of an All Solid Z-Scheme Photocatalyst g-C3N4/GO/AgBr with Enhanced Visible Light Photocatalytic Activity. Appl. Catal. A Gen. 2017, 539, 104–113. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Ismail, A.A. Mesoporous BiVO4/2D-g-C3N4 Heterostructures for Superior Visible Light-Driven Photocatalytic Reduction of Hg(II) Ions. Ceram. Int. 2021, 47, 26063–26073. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Muthamizh, S.; Sureshbabu, K.; Munusamy, S.; Padmanaban, A.; Kaaviya, A.; Nagarajan, R.; Stephen, A.; Narayanan, V. Photocatalytic Properties of Amine Functionalized Bi2Sn2O7/rGO Nanocomposites. J. Phys. Chem. Solids 2018, 118, 21–31. [Google Scholar] [CrossRef]
- Liu, L.; Qi, Y.; Hu, J.; An, W.; Lin, S.; Liang, Y.; Cui, W. Stable Cu2O@g-C3N4 Core@shell Nanostructures: Efficient Visible-Light Photocatalytic Hydrogen Evolution. Mater. Lett. 2015, 158, 278–281. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, Y.; Dong, G.; Wang, C. Br-Doping of g-C3N4 towards Enhanced Photocatalytic Performance in Cr(VI) Reduction. Chin. J. Catal. 2020, 41, 1498–1510. [Google Scholar] [CrossRef]
- Shi, L.; Wang, F.; Sun, J. The Preparation of Spherical Mesoporous g-C3N4 with Highly Improved Photocatalytic Performance for H2 Production and Rhodamine B Degradation. Mater. Res. Bull. 2019, 113, 115–121. [Google Scholar] [CrossRef]
- Xing, Y.; Que, W.; Yin, X.; He, Z.; Liu, X.; Yang, Y.; Shao, J.; Kong, L.B. In2O3/Bi2Sn2O7 Heterostructured Nanoparticles with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2016, 387, 36–44. [Google Scholar] [CrossRef]
- Hu, C.; Zhuang, J.; Zhong, L.; Zhong, Y.; Wang, D.; Zhou, H. Significantly Enhanced Photocatalytic Activity of Visible Light Responsive AgBr/Bi2Sn2O7 Heterostructured Composites. Appl. Surf. Sci. 2017, 426, 1173–1181. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Wang, J.; Zhou, Y.; Wang, J.; Tang, J.; Liu, Y.; Peng, B.; Chen, F. Facile Fabrication of a Direct Z-Scheme Ag2CrO4/g-C3N4 Photocatalyst with Enhanced Visible Light Photocatalytic Activity. J. Mol. Catal. A Chem. 2016, 421, 209–221. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Q.; Tan, K.M.; Wang, F.; Ng, H.Y. Insights into Mechanisms, Kinetics and Pathway of Continuous Visible-Light Photodegradation of PPCPs via Porous g-C3N4 with Highly Dispersed Fe(III) Active Sites. Chem. Eng. J. 2021, 423, 130095. [Google Scholar] [CrossRef]
- Ragupathi, V.; Raja, M.A.; Panigrahi, P.; Ganapathi Subramaniam, N. CuO/g-C3N4 Nanocomposite as Promising Photocatalyst for Photoelectrochemical Water Splitting. Optik 2020, 208, 164569. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, S.; Yu, H.; Quan, X. g-C3N4/TiO2 Hybrid Photocatalyst with Wide Absorption Wavelength Range and Effective Photogenerated Charge Separation. Sep. Purif. Technol. 2012, 99, 50–54. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Yao, J. Shuttle-like CeO2/g-C3N4 Composite Combined with Persulfate for the Enhanced Photocatalytic Degradation of Norfloxacin under Visible Light. Ecotoxicol. Environ. Saf. 2020, 190, 110062. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Prabavathi, S.; Muthuraj, V. Superior Visible Light Driven Photocatalytic Degradation of Fluoroquinolone Drug Norfloxacin over Novel NiWO4 Nanorods Anchored on g-C3N4 Nanosheets. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 43–54. [Google Scholar]
- Zhou, X.; Chen, Y.; Li, C.; Zhang, L.; Zhang, X.; Ning, X.; Zhan, L.; Luo, J. Construction of LaNiO3 Nanoparticles Modified g-C3N4 Nanosheets for Enhancing Visible Light Photocatalytic Activity towards Tetracycline Degradation. Sep. Purif. Technol. 2019, 211, 179–188. [Google Scholar] [CrossRef]
- Zhang, B.; He, X.; Ma, X.; Chen, Q.; Liu, G.; Zhou, Y.; Ma, D.; Cui, C.; Ma, J.; Xin, Y. In Situ Synthesis of Ultrafine TiO2 Nanoparticles Modified g-C3N4 Heterojunction Photocatalyst with Enhanced Photocatalytic Activity. Sep. Purif. Technol. 2020, 247, 116932. [Google Scholar] [CrossRef]
- Xu, Y.; Yifeng, E.; Wang, G. Controlled Growth of “Cookie-like” ZnIn2S4 Nanoparticles on g-C3N4 for Enhanced Visible Light Photocatalytic Activity. Inorg. Chem. Commun. 2019, 108, 107485. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Fang, F.; Yifeng, E.; Zhao, G. Novel Visible-Light-Induced BiOCl/g-C3N4 Photocatalyst for Efficient Degradation of Metronidazole. Inorg. Chem. Commun. 2021, 132, 108820. [Google Scholar] [CrossRef]
- Gao, Y.; Lin, J.; Zhang, Q.; Yu, H.; Ding, F.; Xu, B.; Sun, Y.; Xu, Z. Facile Synthesis of Heterostructured YVO4/g-C3N4/Ag Photocatalysts with Enhanced Visible-Light Photocatalytic Performance. Appl. Catal. B Environ. 2018, 224, 586–593. [Google Scholar] [CrossRef]
- Pan, T.; Chen, D.; Fang, J.; Wu, K.; Feng, W.; Zhu, X.; Fang, Z. Facile Synthesis of Iron and Cerium Co-Doped g-C3N4 with Synergistic Effect to Enhance Visible-Light Photocatalytic Performance. Mater. Res. Bull. 2020, 125, 110812. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Liu, E.; Fan, J. Novel S-Scheme 2D/2D BiOBr/g-C3N4 Heterojunctions with Enhanced Photocatalytic Activity. Chin. J. Catal. 2021, 42, 1519–1529. [Google Scholar] [CrossRef]
- Wang, T.; Quan, W.; Jiang, D.; Chen, L.; Li, D.; Meng, S.; Chen, M. Synthesis of Redox-Mediator-Free Direct Z-Scheme AgI/WO3 Nanocomposite Photocatalysts for the Degradation of Tetracycline with Enhanced Photocatalytic Activity. Chem. Eng. J. 2016, 300, 280–290. [Google Scholar] [CrossRef]
- Jiang, E.; Song, N.; Che, G.; Liu, C.; Dong, H.; Yang, L. Construction of a Z-Scheme MoS2/CaTiO3 Heterostructure by the Morphology-Controlled Strategy towards Enhancing Photocatalytic Activity. Chem. Eng. J. 2020, 399, 125721. [Google Scholar] [CrossRef]
- Luo, J.; Ning, X.; Zhan, L.; Zhou, X. Facile Construction of a Fascinating Z-Scheme AgI/Zn3V2O8 Photocatalyst for the Photocatalytic Degradation of Tetracycline under Visible Light Irradiation. Sep. Purif. Technol. 2021, 255, 117691. [Google Scholar] [CrossRef]
- Wei, K.; Wang, B.; Hu, J.; Chen, F.; Hao, Q.; He, G.; Wang, Y.; Li, W.; Liu, J.; He, Q. Photocatalytic Properties of a New Z-Scheme System BaTiO3/In2S3 with a Core-Shell Structure. RSC Adv. 2019, 9, 11377–11384. [Google Scholar] [CrossRef]
- Jiang, D.; Zhu, Y.; Chen, M.; Huang, B.; Zeng, G.; Huang, D.; Song, B.; Qin, L.; Wang, H.; Wei, W. Modified Crystal Structure and Improved Photocatalytic Activity of MIL-53 via Inorganic Acid Modulator. Appl. Catal. B Environ. 2019, 255, 117746. [Google Scholar] [CrossRef]
- Gao, Z.; Yao, B.; Xu, T.; Ma, M. Effect and Study of Reducing Agent NaBH4 on Bi/BiOBr/CdS Photocatalyst. Mater. Lett. 2020, 259, 126874. [Google Scholar] [CrossRef]
- Che, H.; Liu, C.; Hu, W.; Hu, H.; Li, J.; Dou, J.; Shi, W.; Li, C.; Dong, H. NGQD Active Sites as Effective Collectors of Charge Carriers for Improving the Photocatalytic Performance of Z-Scheme g-C3N4/Bi2WO6 Heterojunctions. Catal. Sci. Technol. 2018, 8, 622–631. [Google Scholar] [CrossRef]
- Hao, C.C.; Tang, Y.B.; Shi, W.L.; Chen, F.Y.; Guo, F. Facile Solvothermal Synthesis of a Z-Scheme 0D/3D CeO2/ZnIn2S4 Heterojunction with Enhanced Photocatalytic Performance under Visible Light Irradiation. Chem. Eng. J. 2021, 409, 128168. [Google Scholar] [CrossRef]
- Zhu, Z.; Xia, H.; Wu, R.; Cao, Y.; Li, H. Fabrication of La2O3/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance of Tetracycline Hydrochloride. Crystals 2021, 11, 1349. [Google Scholar] [CrossRef]
- Tian, Q.; Zhuang, J.; Wang, J.; Xie, L.; Liu, P. Novel Photocatalyst, Bi2Sn2O7, for Photooxidation of As(III) under Visible-Light Irradiation. Appl. Catal. A Gen. 2012, 425–426, 74–78. [Google Scholar] [CrossRef]
- Yaghoot-Nezhad, A.; Moradi, M.; Rostami, M.; Danaee, I.; Khosravi-Nikou, M.R. Dual Z-Scheme CuO-ZnO@Graphitic Carbon Nitride Ternary Nanocomposite with Improved Visible Light-Induced Catalytic Activity for Ultrasound-Assisted Photocatalytic Desulfurization. Energy Fuels 2020, 34, 13588–13605. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Xia, H.; Li, H.; Han, S. Facile Construction of Bi2Sn2O7/g-C3N4 Heterojunction with Enhanced Photocatalytic Degradation of Norfloxacin. Inorganics 2022, 10, 131. https://doi.org/10.3390/inorganics10090131
Zhu Z, Xia H, Li H, Han S. Facile Construction of Bi2Sn2O7/g-C3N4 Heterojunction with Enhanced Photocatalytic Degradation of Norfloxacin. Inorganics. 2022; 10(9):131. https://doi.org/10.3390/inorganics10090131
Chicago/Turabian StyleZhu, Zhengru, Haiwen Xia, Hong Li, and Songlin Han. 2022. "Facile Construction of Bi2Sn2O7/g-C3N4 Heterojunction with Enhanced Photocatalytic Degradation of Norfloxacin" Inorganics 10, no. 9: 131. https://doi.org/10.3390/inorganics10090131