Elasticity Changes in the Crystalline Lens during Oxidative Damage and the Antioxidant Effect of Alpha-Lipoic Acid Measured by Optical Coherence Elastography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
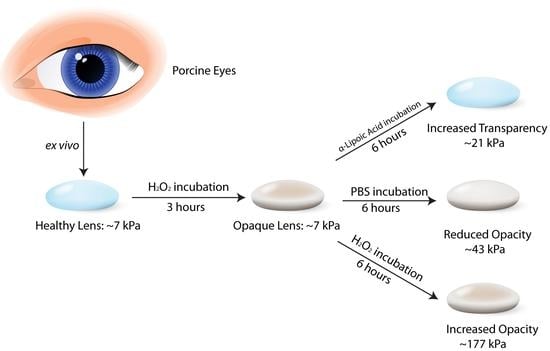
2.2. Sample Preparation
2.3. Optical Properties Measurements
2.4. Surface Wave Measurements
3. Results
3.1. Lens Transparency Analysis
3.2. OCE Measurement of the Lenticular Biomechanical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asbell, P.A.; Dualan, L.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendschot, T.T.; Broekmans, W.M.; Klopping-Ketelaars, I.A.; Kardinaal, A.F.; Van Poppel, G.; Van Norren, D. Lens aging in relation to nutritional determinants and possible risk factors for age-related cataract. Arch. Ophthalmol. 2002, 120, 1732–1737. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Kumar, D.; Singh, R. Phytotherapeutics for management and prevention of cataract generation. Phytopharmacology 2012, 3, 93–110. [Google Scholar]
- Beebe, D.C.; Holekamp, N.M.; Shui, Y.-B. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010, 44, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.-L.; Lou, M. The effect and recovery of long-term H2O2 exposure on lens morphology and biochemestry. Exp. Eye Res. 1993, 57, 157–167. [Google Scholar] [CrossRef]
- McGinty, S.J.; Truscott, R.J.W. Presbyopia: The first stage of nuclear cataract? Ophthalmic Res. 2006, 38, 137–148. [Google Scholar] [CrossRef]
- Michael, R.; Bron, A.J. The ageing lens and cataract: A model of normal and pathological ageing. Philos. Trans. R. Soc. B 2011, 366, 1278–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.; Davies, K.J.A. Protein oxidation and loss of protease activity may lead to cataract formation in the aged lens. Free Radic. Biol. Med. 1987, 3, 371–377. [Google Scholar] [CrossRef]
- Truscott, R.J. Age-related nuclear cataract—Oxidation is the key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- Dilley, K.J.; Pirie, A. Changes to the proteins of the human lens nucleus in cataract. Exp. Eye Res. 1974, 19, 59–72. [Google Scholar] [CrossRef]
- Kleiman, N.J.; Spector, A. DNA single-strand breaks in human lens epithelial-cells from patients with cataract. Curr. Eye Res. 1993, 12, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Heruye, S.H.; Nkenyi, L.N.M.; Singh, N.U.; Yalzadeh, D.; Ngele, K.K.; Njie-Mbye, Y.-F.; Ohia, S.E.; Opere, C.A. Current trends in the pharmacotherapy of cataracts. Pharmaceuticals 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamei, A. Glutathione levels of the human crystalline lens in aging and its antioxidant effect against the oxidation of lens proteins. Biol. Pharm. Bull. 1993, 16, 870–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, W.H.; Garner, M.H. Protein disulfide levels and lens elasticity modulation: Applications for presbyopia. Invest. Ophthalmol. Vis. Sci. 2016, 57, 2851–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, L.; Mao, J.; Reitz, J.; Sreeramulu, S.; Kudlinzki, D.; Hodirnau, V.V.; Meier-Credo, J.; Saxena, K.; Buhr, F.; Langer, J.D.; et al. Cysteine oxidation and disulfide formation in the ribosomal exit tunnel. Nat. Commun. 2020, 11, 5569. [Google Scholar] [CrossRef] [PubMed]
- Giblin, F.J.; Chakrapani, B.; Reddy, V.N. Glutathione and lens epithelial function. Invest. Ophthalmol. 1976, 15, 381–393. [Google Scholar] [PubMed]
- Giblin, F.J.; Mccready, J.P.; Reddan, J.R.; Dziedzic, D.C.; Reddy, V.N. Detoxification of H2O2 by cultured rabbit lens epithelial-cells—Participation of the glutathione redox cycle. Exp. Eye Res. 1985, 40, 827–840. [Google Scholar] [CrossRef]
- Williams, D.L. Oxidation, antioxidants and cataract formation: A literature review. Vet. Sci. 2006, 9, 292–298. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Kan, E.; Kiliçkan, E.; Ayar, A.; Çolak, R. Effects of two antioxidants; a-lipoic acid and fisetin against diabetic cataract in mice. Int. Ophthalmol. 2015, 35, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.B.; Choudhary, R.; Vishwakarma, P.K.; Singh, A.; Shree, J.; Bodakhe, S.H. Protective effect of alpha-lipoic acid on progression of cataract formation in fructose-induced experimental cataract. Pharma. Nutr. 2017, 5, 127–132. [Google Scholar] [CrossRef]
- Kojima, M.; Sun, L.; Hata, I.; Sakamoto, Y.; Sasaki, H.; Sasaki, K. Efficacy of a-lipoic acid against diabetic cataract in rat. Jpn. J. Ophthalmol. 2007, 51, 10–13. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.-Z.; Shi, J.-M.; Jia, S.-B. Alpha lipoic acid protects lens from H2O2-induced cataract by inhibiting apoptosis of lens epithelial cells and inducing activation of anti-oxidative enzymes. Asian Pac. J. Trop. Med. 2013, 6, 548–551. [Google Scholar] [CrossRef] [Green Version]
- Maitra, I.; Serbinova, E.; Trischler, H.; Packer, L. A-lipoic acid prevents buthionine sulfoximine-induced cataract formation in newborn rats. Free Radic. Biol. Med. 1995, 18, 823–829. [Google Scholar] [CrossRef]
- Williams, D.L. Effect of oral alpha lipoic acid in preventing the genesis of canine diabetic cataract: A preliminary study. Vet. Sci. 2017, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krag, S.; Andreassen, T.T. Biomechanical measurements of the porcine lens capsule. Exp. Eye Res. 1996, 62, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Weeber, H.A.; Eckert, G.; Pechhold, W.; van der Heijde, R.G. Stiffness gradient in the crystalline lens. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Weeber, H.A.; Eckert, G.; Soergel, F.; Meyer, C.H.; Pechhold, W.; van der Heijde, R.G. Dynamic mechanical properties of human lenses. Exp. Eye Res. 2005, 80, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Heys, K.R.; Truscott, R.J.W. The stiffness of human cataract lenses is a function of both age and the type of cataract. Exp. Eye Res. 2008, 86, 701–703. [Google Scholar] [CrossRef]
- Hollman, K.W.; O’Donnell, M.; Erpelding, T.N. Mapping elasticity in human lenses using bubble-based acoustic radiation force. Exp. Eye Res. 2007, 85, 890–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.; Aglyamov, S.; Karpiouk, A.; Emelianov, S. A high pulse repetition frequency ultrasound system for the ex vivo measurement of mechanical properties of crystalline lenses with laser-induced microbubbles interrogated by acoustic radiation force. Phys. Med. Biol. 2012, 57, 4871–4884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.; Aglyamov, S.; Karpiouk, A.; Emelianov, S. The mechanical properties of ex vivo bovine and porcine crystalline lenses: Age-related changes and location-dependent variations. Ultrasound Med. Biol. 2013, 39, 1120–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simsek, C.; Gokmen, O. The effects of vital dyes on mechanical properties of the human anterior lens capsule. Indian J. Ophthalmol. 2020, 68, 66–70. [Google Scholar]
- Scarcelli, G.; Kim, P.; Yun, S.H. In vivo measurement of age-related stiffening in the crystalline lens by brillouin optical microscopy. Biophys. J. 2011, 101, 1539–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambekar, Y.S.; Singh, M.; Zhang, J.; Nair, A.; Aglyamov, S.R.; Scarcelli, G.; Larin, K.V. Multimodal quantitative optical elastography of the crystalline lens with optical coherence elastography and brillouin microscopy. Biomed. Opt. Express 2020, 11, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Detorakis, E.T.; Drakonaki, E.E.; Ginis, H.; Karyotakis, N.; Pallikaris, I.G. Evaluation of iridociliary and lenticular elasticity using shear-wave elastography in rabbit eyes. Acta Med. 2014, 57, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Yoon, H.; Larin, K.V.; Emelianov, S.Y.; Aglyamov, S.R. The impact of intraocular pressure on elastic wave velocity estimates in the crystalline lens. Phys. Med. Biol. 2016, 62, N45–N57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Lyu, Z.; Gao, X.; Zhang, P.; Lin, H.; Guo, Y.; Wang, T.; Chen, S.; Chen, X. Noninvasive assessment of age-related stiffness of crystalline lenses in a rabbit model using ultrasound elastography. Biomed. Eng. Online 2018, 17, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.Y.; Yan, H.; Yan, W.J.; Wang, X.C.; Li, Q.Y. Noninvasive stiffness assessment of the human lens nucleus in patients with anisometropia using an ultrasound elastography system. Int. J. Ophthalmol. 2020, 13, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.; Chen, J.J.; Yu, J.; Jin, Z.; Miao, Y.; Browne, A.W.; Zhou, Q.; Chen, Z. Simultaneously imaging and quantifying in vivo mechanical properties of crystalline lens and cornea using optical coherence elastography with acoustic radiation force excitation. APL Photonics 2019, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Moon, S.; Chen, J.J.; Zhu, Z.K.; Chen, Z.P. Ultrahigh-sensitive optical coherence elastography. Light Sci. Appl. 2020, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Aglyamov, S.R.; Han, Z.L.; Singh, M.; Liu, C.H.; Larin, K.V. Assessing the biomechanical properties of the porcine crystalline lens as a function of intraocular pressure with optical coherence elastography. Biomed. Opt. Express 2018, 9, 6455–6466. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Aglyamov, S.R.; Zhang, H.; Larin, K.V. Measuring the elastic wave velocity in the lens of the eye as a function of intraocular pressure using optical coherent elastography. Quantum Electron. 2019, 49, 20–24. [Google Scholar] [CrossRef]
- Larin, K.V.; Sampson, D.D. Optical coherence elastography—OCT at work in tissue biomechanics [invited]. Biomed. Opt. Express 2017, 8, 1172–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Han, Z.; Wang, S.; Li, J.; Singh, M.; Liu, C.H.; Aglyamov, S.; Emelianov, S.; Manns, F.; Larin, K.V. Assessing age-related changes in the biomechanical properties of rabbit lens using a coaligned ultrasound and optical coherence elastography system. Invest. Ophthalmol. Vis. Sci. 2015, 56, 1292–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wu, C.; Singh, M.; Nair, A.; Aglyamov, S.; Larin, K. Optical coherence elastography of cold cataract in porcine lens. J. Biomed. Opt. 2019, 24, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, M.; Augusteyn, R.C. The effects of hydrogen peroxide on lens proteins: A possible model for nuclear cataract. Exp. Eye Res. 1984, 38, 45–56. [Google Scholar] [CrossRef]
- Carbajal, E.F.; Baranov, S.A.; Manne, V.G.; Young, E.D.; Lazar, A.J.; Lev, D.C.; Pollock, R.E.; Larin, K.V. Revealing retroperitoneal liposarcoma morphology using optical coherence tomography. J. Biomed. Opt. 2011, 16, 020502. [Google Scholar] [CrossRef]
- Wang, S.; Larin, K.V.; Li, J.S.; Vantipalli, S.; Manapuram, R.K.; Aglyamov, S.; Emelianov, S.; Twa, M.D. A focused air-pulse system for optical-coherence-tomography-based measurements of tissue elasticity. Laser Phys. Lett. 2013, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Huang, Z.; Wang, R.K. Tracking mechanical wave propagation within tissue using phase-sensitive optical coherence tomography: Motion artifact and its compensation. J. Biomed. Opt. 2013, 18, 121505. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, C.H.; Zakharov, V.P.; Lazar, A.J.; Pollock, R.E.; Larin, K.V. Three-dimensional computational analysis of optical coherence tomography images for the detection of soft tissue sarcomas. J. Biomed. Opt. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, J.; Singh, M.; Wu, C.; Liu, C.-h.; Wang, S.; Idugboe, R.; Raghunathan, R.; Sudheendran, N.; Aglyamov, S.R. Quantitative methods for reconstructing tissue biomechanical properties in optical coherence elastography: A comparison study. Phys. Med. Biol. 2015, 60, 3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, J.F. Wave propagation in structure: Spectral analysis using fast discrete Fourier transforms. In Mechanical Engineering Series; Springer-Verlag: New York, NY, USA, 1997. [Google Scholar]
- Vilupuru, A.S.; Glasser, A. Optical and biometric relationships of the isolated pig crystalline lens. Ophthalmic Physiol. Opt. 2001, 21, 296–311. [Google Scholar] [CrossRef] [Green Version]
- Ozgokce, M.; Batur, M.; Alpaslan, M.; Yavuz, A.; Batur, A.; Seven, E.; Arslan, H. A comparative evaluation of cataract classifications based on shear-wave elastography and b-mode ultrasound findings. J. Ultrasound 2019, 22, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.D.; Hegde, K.R.; Kovtun, S. Oxidative damage to lens in culture: Reversibility by pyruvate and ethyl pyruvate. Ophthalmologica 2006, 220, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.; Wang, G.M.; Wang, R.R.; Garner, W.H.; Moll, H. The prevention of cataract caused by oxidative stress in cultured rat lenses. I. H2O2 and photochemically induced cataract. Curr. Eye Res. 1993, 12, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Schachar, R.A.; Chan, R.W.; Fu, M. Viscoelastic shear properties of the fresh porcine lens. Br. J. Ophthalmol. 2007, 91, 366–368. [Google Scholar] [CrossRef] [Green Version]






| 0 h | 3 h | 6 h | 9 h | |||||
|---|---|---|---|---|---|---|---|---|
| cg (m/s) | E (kPa) | cg (m/s) | E (kPa) | cg (m/s) | E (kPa) | cg (m/s) | E (kPa) | |
| H2O2 | 1.47 ± 0.22 | 7.28 ± 2.29 | 1.26 ± 0.06 | 5.21 ± 0.51 | 2.34 ± 0.08 | 18.09 ± 1.33 | 7.26 ± 0.90 | 176.62 ± 44.86 |
| H2O2 → ALA | 1.25 ± 0.07 | 5.15 ± 0.60 | 1.41 ± 0.03 | 6.55 ± 0.25 | 1.62 ± 0.05 | 8.69 ± 0.55 | 2.49 ± 0.21 | 20.67 ± 3.51 |
| H2O2 → PBS | 1.39 ± 0.17 | 6.44 ± 1.61 | 1.49 ± 0.15 | 7.35 ± 1.51 | 2.29 ± 0.16 | 18.08 ± 2.87 | 3.61 ± 0.40 | 43.03 ± 8.99 |
| ALA | 1.44 ± 0.18 | 6.95 ± 1.80 | 1.34 ± 0.06 | 5.98 ± 0.51 | 1.36 ± 0.05 | 6.12 ± 0.44 | 1.51 ± 0.31 | 7.82 ± 3.38 |
| PBS | 1.56 ± 0.09 | 8.18 ± 1.06 | 1.28 ± 0.18 | 5.54 ± 1.47 | 1.25 ± 0.10 | 5.17 ± 0.78 | 1.37 ± 0.14 | 6.28 ± 1.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Singh, M.; Nair, A.; Larin, K.V.; Aglyamov, S.R. Elasticity Changes in the Crystalline Lens during Oxidative Damage and the Antioxidant Effect of Alpha-Lipoic Acid Measured by Optical Coherence Elastography. Photonics 2021, 8, 207. https://doi.org/10.3390/photonics8060207
Zhang H, Singh M, Nair A, Larin KV, Aglyamov SR. Elasticity Changes in the Crystalline Lens during Oxidative Damage and the Antioxidant Effect of Alpha-Lipoic Acid Measured by Optical Coherence Elastography. Photonics. 2021; 8(6):207. https://doi.org/10.3390/photonics8060207
Chicago/Turabian StyleZhang, Hongqiu, Manmohan Singh, Achuth Nair, Kirill V. Larin, and Salavat R. Aglyamov. 2021. "Elasticity Changes in the Crystalline Lens during Oxidative Damage and the Antioxidant Effect of Alpha-Lipoic Acid Measured by Optical Coherence Elastography" Photonics 8, no. 6: 207. https://doi.org/10.3390/photonics8060207