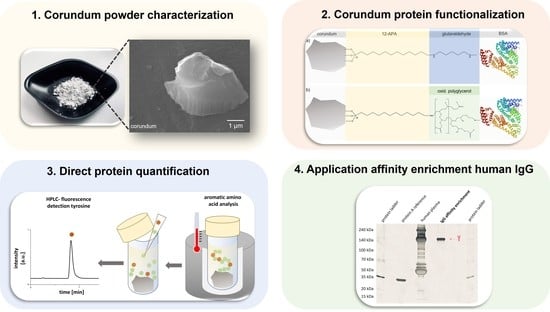
High-Purity Corundum as Support for Affinity Extractions from Complex Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TEM, ESEM
2.3. Dynamic Light Scattering and Zeta Potential Measurements
2.4. BET
2.5. H NMR of Polyglycerol (PG) and Oxidized Polyglycerol
2.6. Purification Protocol for Raw Corundum
2.7. Functionalization of Corundum with 12-Amino Dodecyl Phosphonic Acid Hydrochloride (12-APA) and Glutaraldehyde or Oxidized Polyglycerol for the Conjugation with BSA or Protein A
2.8. Aromatic Amino Acid Analysis and HPLC Analysis
2.9. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and IgG Affinity Enrichment
3. Results and Discussion
3.1. Corundum Particle Characterization
3.2. Surface Functionalization with Amino Phosphonic Acids, Activated with Glutaraldehyde or Oxidized Hyperbranched Polyglycerol
3.3. Protein Immobilization and Direct Quantification with Aromatic Amino Acid Analysis (AAAA)
3.4. Affinity Enrichment of IgG from Human Plasma with Protein-A-Functionalized Corundum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneck, N.A.; Phinney, K.W.; Lee, S.B.; Lowenthal, M.S. Quantification of cardiac troponin I in human plasma by immunoaffinity enrichment and targeted mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Makawita, S.; Diamandis, E.P. The bottleneck in the cancer biomarker pipeline and protein quantification through mass spectrometry–based approaches: Current strategies for candidate verification. Clin. Chem. 2010, 56, 212–222. [Google Scholar] [CrossRef]
- Sauer, P.W.; Burky, J.E.; Wesson, M.C.; Sternard, H.D.; Qu, L. A high-yielding, generic fed-batch cell culture process for production of recombinant antibodies. Biotechnol. Bioeng. 2000, 67, 585–597. [Google Scholar] [CrossRef]
- Aires da Silva, F.; Corte-Real, S.; Goncalves, J. Recombinant antibodies as therapeutic agents. Biodrugs 2008, 22, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Łącki, K.M.; Riske, F.J. Affinity Chromatography: An Enabling Technology for Large-Scale Bioprocessing. Biotechnol. J. 2020, 15, 1800397. [Google Scholar] [CrossRef] [PubMed]
- Groman, E.V.; Wilchek, M. Recent developments in affinity chromatography supports. Trends Biotechnol. 1987, 5, 220–224. [Google Scholar] [CrossRef]
- Cuatrecasas, P.; Wilchek, M.; Anfinsen, C.B. Selective enzyme purification by affinity chromatography. Proc. Natl. Acad. Sci. USA 1968, 61, 636–643. [Google Scholar] [CrossRef]
- Göke, B.; Keim, V. HPLC and FPLC. Int. J. Pancreatol. 1992, 11, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Whiteaker, J.R.; Zhao, L.; Zhang, H.Y.; Feng, L.-C.; Piening, B.D.; Anderson, L.; Paulovich, A.G. Antibody-based enrichment of peptides on magnetic beads for mass-spectrometry-based quantification of serum biomarkers. Anal. Biochem. 2007, 362, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Changhe, L.; Zhang, Y.; Wenfeng, D.; Min, Y.; Yuying, Y.; Han, Z.; Xuefeng, X.; Dazhong, W.; Debnath, S. Advances in fabrication of ceramic corundum abrasives based on sol–gel process. Chin. J. Aeronaut. 2021, 34, 1–17. [Google Scholar] [CrossRef]
- Selvakumaran, D.; Arunachalam, N.; Vijayaraghavan, L.; Balan, A. Performance comparison of sol-gel with white alumina abrasives for grinding of super duplex stainless steel (SDSS). Procedia Manuf. 2018, 26, 1448–1458. [Google Scholar] [CrossRef]
- Krell, A.; Blank, P.; Wagner, E.; Bartels, G. Advances in the grinding efficiency of sintered alumina abrasives. J. Am. Ceram. Soc. 1996, 79, 763–769. [Google Scholar] [CrossRef]
- Nadolny, K. State of the art in production, properties and applications of the microcrystalline sintered corundum abrasive grains. Int. J. Adv. Manuf. Technol. 2014, 74, 1445–1457. [Google Scholar] [CrossRef]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.; Zuilhof, H. Covalent surface modification of oxide surfaces. Angew. Chem. Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef]
- Luschtinetz, R.; Oliveira, A.F.; Duarte, H.A.; Seifert, G. Self-assembled Monolayers of Alkylphosphonic Acids on Aluminum Oxide Surfaces–A Theoretical Study. Z. Anorg. Allg. Chem. 2010, 636, 1506–1512. [Google Scholar] [CrossRef]
- Messerschmidt, C.; Schwartz, D.K. Growth mechanisms of octadecylphosphonic acid self-assembled monolayers on sapphire (corundum): Evidence for a quasi-equilibrium triple point. Langmuir 2001, 17, 462–467. [Google Scholar] [CrossRef]
- Bauer, T.; Schmaltz, T.; Lenz, T.; Halik, M.; Meyer, B.; Clark, T. Phosphonate-and carboxylate-based self-assembled monolayers for organic devices: A theoretical study of surface binding on aluminum oxide with experimental support. ACS Appl. Mater. Interfaces 2013, 5, 6073–6080. [Google Scholar] [CrossRef]
- Thissen, P.; Valtiner, M.; Grundmeier, G. Stability of phosphonic acid self-assembled monolayers on amorphous and single-crystalline aluminum oxide surfaces in aqueous solution. Langmuir 2010, 26, 156–164. [Google Scholar] [CrossRef]
- Frasconi, M.; Mazzei, F.; Ferri, T. Protein immobilization at gold–thiol surfaces and potential for biosensing. Anal. Bioanal. Chem. 2010, 398, 1545–1564. [Google Scholar] [CrossRef]
- Silin, V.; Weetall, H.; Vanderah, D.J. SPR studies of the nonspecific adsorption kinetics of human IgG and BSA on gold surfaces modified by self-assembled monolayers (SAMs). J. Colloid Interface Sci. 1997, 185, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.D.; Whitesides, G.M. Modeling organic surfaces with self-assembled monolayers. Angew. Chem. 1989, 101, 522–528. [Google Scholar] [CrossRef]
- Klykov, O.; Weller, M.G. Quantification of N-hydroxysuccinimide and N-hydroxysulfosuccinimide by hydrophilic interaction chromatography (HILIC). Anal. Methods 2015, 7, 6443–6448. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Clinton, F. Sodium cyanoborohydride—A highly selective reducing agent for organic functional groups. Synthesis 1975, 3, 135–146. [Google Scholar] [CrossRef]
- Zeiger, E.; Gollapudi, B.; Spencer, P. Genetic toxicity and carcinogenicity studies of glutaraldehyde—A review. Mutat. Res./Rev. Mutat. Res. 2005, 589, 136–151. [Google Scholar] [CrossRef]
- Beauchamp, R.O.; St Clair, M.B.G.; Fennell, T.R.; Clarke, D.O.; Morgan, K.T.; Karl, F.W. A critical review of the toxicology of glutaraldehyde. Crit. Rev. Toxicol. 1992, 22, 143–174. [Google Scholar] [CrossRef]
- Calderón, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic polyglycerols for biomedical applications. Adv. Mater. 2010, 22, 190–218. [Google Scholar] [CrossRef]
- Unbehauen, M.L.; Fleige, E.; Paulus, F.; Schemmer, B.; Mecking, S.; Moré, S.D.; Haag, R. Biodegradable core–multishell nanocarriers: Influence of inner shell structure on the encapsulation behavior of dexamethasone and tacrolimus. Polymers 2017, 9, 316. [Google Scholar] [CrossRef]
- Pant, K.; Groger, D.; Bergmann, R.; Pietzsch, J.; Steinbach, J.; Graham, B.; Spiccia, L.; Berthon, F.; Czarny, B.; Devel, L. Synthesis and biodistribution studies of 3H-and 64Cu-labeled dendritic polyglycerol and dendritic polyglycerol sulfate. Bioconjug. Chem. 2015, 26, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.; Haag, R. Dendritic polyglycerol: A new versatile biocompatible material. Rev. Mol. Biotechnol. 2002, 90, 257–267. [Google Scholar] [CrossRef]
- Höger, K.; Becherer, T.; Qiang, W.; Haag, R.; Frieß, W.; Küchler, S. Polyglycerol coatings of glass vials for protein resistance. Eur. J. Pharm. Biopharm. 2013, 85, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Frey, H. Controllable nonspecific protein adsorption by charged hyperbranched polyglycerol thin films. Langmuir 2015, 31, 13101–13106. [Google Scholar] [CrossRef]
- Moore, E.; Delalat, B.; Vasani, R.; Thissen, H.; Voelcker, N.H. Patterning and biofunctionalization of antifouling hyperbranched polyglycerol coatings. Biomacromolecules 2014, 15, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Wu, T.; Chen, Q.; Lian, X.; Wu, H.; Shi, B. Hyperbranched polyglycerols as robust up-conversion nanoparticle coating layer for feasible cell imaging. Polymers 2020, 12, 2592. [Google Scholar] [CrossRef]
- Jones, G. Polymerization of glutaraldehyde at fixative pH. J. Histochem. Cytochem. 1974, 22, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Rembaum, A.; Margel, S.; Levy, J. Polyglutaraldehyde: A new reagent for coupling proteins to microspheres and for labeling cell-surface receptors. J. Immunol. Methods 1978, 24, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Moks, T.; Abrahmsén, L.; Nilsson, B.; Hellman, U.; Sjöquist, J.; Uhlén, M. Staphylococcal protein A consists of five IgG-binding domains. Eur. J. Biochem. 1986, 156, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Pecher, H.S.; Zimathies, A.; Weller, M.G. Oligoepoxide-Based Monoliths: Synthesis and Application as Affinity Capillary Column for Enrichment of Immunoglobulin G. Macromol. Chem. Phys. 2012, 213, 2398–2403. [Google Scholar] [CrossRef]
- Wilke, M.; Röder, B.; Paul, M.; Weller, M.G. Sintered glass monoliths as supports for affinity columns. Separations 2021, 8, 56. [Google Scholar] [CrossRef]
- Nayak, N.; Mazzei, R.; Giorno, L.; Crespo, J.G.; Portugal, C.A.; Poerio, T. Protein Attachment Mechanism for Improved Functionalization of Affinity Monolith Chromatography (AMC). Molecules 2022, 27, 4496. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Hesse, A.; Weller, M.G. Protein quantification by derivatization-free high-performance liquid chromatography of aromatic amino acids. J. Amino Acids 2016, 2016, 7374316. [Google Scholar] [CrossRef] [PubMed]
- Reinmuth-Selzle, K.; Tchipilov, T.; Backes, A.T.; Tscheuschner, G.; Tang, K.; Ziegler, K.; Lucas, K.; Pöschl, U.; Fröhlich-Nowoisky, J.; Weller, M.G. Determination of the protein content of complex samples by aromatic amino acid analysis, liquid chromatography-UV absorbance, and colorimetry. Anal. Bioanal. Chem. 2022, 414, 4457–4470. [Google Scholar] [CrossRef] [PubMed]
- Image from the Protein Data Bank (rcsb.org) PDB ID 4F5S. Bujacz, A.; Bujacz, G. Crystal Structure of Bovine Serum Albumin. 2012. Available online: https://www.wwpdb.org/pdb?id=pdb_00004f5s (accessed on 30 August 2022). [CrossRef]
- Tchipilov, T.; Raysyan, A.; Weller, M.G. Methods for the quantification of particle-bound protein—Application to reagents for lateral-flow immunoassays (LFIA). Preprints 2022, 2022030332. [Google Scholar] [CrossRef]
- Sousa, M.M.; Steen, K.W.; Hagen, L.; Slupphaug, G. Antibody cross-linking and target elution protocols used for immunoprecipitation significantly modulate signal-to noise ratio in downstream 2D-PAGE analysis. Proteome Sci. 2011, 9, 1–8. [Google Scholar] [CrossRef]
- Pernemalm, M.; Sandberg, A.; Zhu, Y.; Boekel, J.; Tamburro, D.; Schwenk, J.M.; Björk, A.; Wahren-Herlenius, M.; Åmark, H.; Östenson, C.-G. In-depth human plasma proteome analysis captures tissue proteins and transfer of protein variants across the placenta. Elife 2019, 8, e41608. [Google Scholar] [CrossRef] [PubMed]





| Sample | Zeta Potential [mV] |
|---|---|
| Corundum | 4.28 ± 0.01 |
| Corundum-12-APA | −2.94 ± 0.37 |
| Corundum-12-APA-glutaraldehyde | −15.00 ± 0.52 |
| Corundum-12-APA-oxidized polyglycerol | −15.10 ± 3.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Völzke, J.L.; Shamami, P.H.; Gawlitza, K.; Feldmann, I.; Zimathies, A.; Meyer, K.; Weller, M.G. High-Purity Corundum as Support for Affinity Extractions from Complex Samples. Separations 2022, 9, 252. https://doi.org/10.3390/separations9090252
Völzke JL, Shamami PH, Gawlitza K, Feldmann I, Zimathies A, Meyer K, Weller MG. High-Purity Corundum as Support for Affinity Extractions from Complex Samples. Separations. 2022; 9(9):252. https://doi.org/10.3390/separations9090252
Chicago/Turabian StyleVölzke, Jule L., Parya Hodjat Shamami, Kornelia Gawlitza, Ines Feldmann, Annett Zimathies, Klas Meyer, and Michael G. Weller. 2022. "High-Purity Corundum as Support for Affinity Extractions from Complex Samples" Separations 9, no. 9: 252. https://doi.org/10.3390/separations9090252