Mesoporous Activated Carbon from Bamboo Waste via Microwave-Assisted K2CO3 Activation: Adsorption Optimization and Mechanism for Methylene Blue Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbent
2.3. Characterization Techniques
2.4. Design of Experiments
3. Results and Discussion
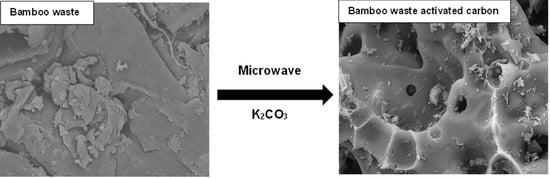
3.1. Characterization of Adsorbent
3.2. Optimization Study
3.2.1. Regression Model Improvement
3.2.2. Analysis of Variance (ANOVA)
3.2.3. Interactive Effects on MB Removal
3.2.4. Model Validation and Desirability Function for MB Removal
3.3. Adsorption Experiments
3.4. Adsorption Kinetics
3.5. Adsorption Isotherm
3.6. Thermodynamics Study
3.7. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2022, 4, 100209. [Google Scholar] [CrossRef]
- Obinnaa, I.B.; Ebere, E.C. A Review: Water pollution by heavy metal and organic pollutants: Brief review of sources, effects and progress on remediation with aquatic plants. Anal. Methods Environ. Chem. J. 2019, 6, 5–38. [Google Scholar] [CrossRef] [Green Version]
- Sah, M.K.; Edbey, K.; EL-Hashani, A.; Almshety, S.; Mauro, L.; Alomar, T.S.; AlMasoud, N.; Bhattarai, A. Exploring the biosorption of methylene blue dye onto agricultural products: A critical review. Separations 2022, 9, 256. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S. A review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.N. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.; Ma, X.; Du, Q.; Sui, K.; Wang, D.; Wang, C. Filtration and adsorption properties of porous calcium alginate membrane for methylene blue removal from water. Chem. Eng. J. 2017, 316, 623–630. [Google Scholar] [CrossRef]
- El-Moselhy, M.M.; Kamal, S.M. Selective removal and preconcentration of methylene blue from polluted water using cation exchange polymeric material. Groundw. Sustain. Dev. 2018, 6, 6–13. [Google Scholar] [CrossRef]
- Aouaini, F.; Dhaouadi, F.; Sellaoui, L.; Badawi, M.; Bonilla-Petriciolet, A.; Lamine, A.B. Using an enhanced multilayer model to analyze the performance of nickel alginate/graphene oxide aerogel, nickel alginate aerogel/activated carbon, and activated carbon in the adsorption of a textile dye pollutant. Environ. Sci. Pollut. Res. 2022, 29, 63622–63628. [Google Scholar] [CrossRef]
- Li, H.; Lin, Y.; Luo, Y.; Yu, P.; Hou, L. Relating organic fouling of reverse osmosis membranes to adsorption during the reclamation of secondary effluents containing methylene blue and rhodamine B. J. Hazard. Mater. 2011, 192, 490–499. [Google Scholar] [CrossRef]
- Dinh, V.-P.; Tran, N.Q.; Tran, Q.-H.; Nguyen, T.D. Facile synthesis of FeFe2O4 magnetic nanomaterial for removing methylene blue from aqueous solution. Prog. Nat. Sci. USA 2019, 29, 648–654. [Google Scholar] [CrossRef]
- Durrani, W.Z.; Nasrullah, A.; Khan, A.S.; Fagieh, T.M.; Bakhsh, E.M.; Akhtar, K.; Khan, S.B. Adsorption efficiency of date palm based activated carbon-alginate membrane for methylene blue. Chemosphere 2022, 302, 134793. [Google Scholar] [CrossRef] [PubMed]
- Saghi, M.H.; Qasemi, M.; Alidadi, H.; Alahabadi, A.; Rastegar, A.; Kowsari, M.H.; Shams, M. Vanadium oxide nanoparticles for methylene blue water remediation: Exploring the effect of physicochemical parameters by process modeling. J. Mol. Liq. 2020, 318, 114046. [Google Scholar] [CrossRef]
- Pradhan, A.C.; Paul, A.; Rao, G.R. Sol-gel-cum-hydrothermal synthesis of mesoporous Co-Fe@ Al2O3−MCM-41 for methylene blue remediation. J. Chem. Sci. 2017, 129, 381–395. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Zeng, G.; Chen, A.; Huang, Z.; Peng, M.; Huang, T.; Chen, G. Graphene hybridized polydopamine-kaolin composite as effective adsorbent for methylene blue removal. Compos. B Eng. 2019, 161, 141–149. [Google Scholar] [CrossRef]
- Mustafa, I. Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem. J. 2019, 144, 397–402. [Google Scholar]
- Jawad, A.H.; Sahu, U.K.; Mastuli, M.S.; ALOthman, Z.A.; Wilson, L.D. Multivariable optimization with desirability function for carbon porosity and methylene blue adsorption by watermelon rind activated carbon prepared by microwave assisted H3PO4. Biomass Convers. Biomass Convers. Biorefinery 2022, 1–15. [Google Scholar] [CrossRef]
- Gupta, S.A.; Vishesh, Y.; Sarvshrestha, N.; Bhardwaj, A.S.; Kumar, P.A.; Topare, N.S.; Raut-Jadhav, S. Adsorption isotherm studies of methylene blue using activated carbon of waste fruit peel as an adsorbent. Mater. Today 2022, 57, 1500–1508. [Google Scholar] [CrossRef]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Lamine, A.B. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. J. Chem. Eng. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; El-Chaghaby, G.A.; ElGammal, M.H.; Rawash, E.S.A. Optimizing the preparation conditions of activated carbons from olive cake using KOH activation. New Carbon Mater. 2016, 31, 492–500. [Google Scholar] [CrossRef]
- Deng, H.; Li, G.; Yang, H.; Tang, J.; Tang, J. Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3 activation. Chem. Eng. J. 2010, 163, 373–381. [Google Scholar] [CrossRef]
- Cansado, I.P.D.P.; Belo, C.R.; Mourao, P.A.M. Pesticides abatement using activated carbon produced from a mixture of synthetic polymers by chemical activation with KOH and K2CO3. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100261. [Google Scholar]
- Saygılı, H.; Saygılı, G.A. Optimized preparation for bimodal porous carbon from lentil processing waste by microwave-assisted K2CO3 activation: Spectroscopic characterization and dye decolorization activity. J. Clean. Prod. 2019, 226, 968–976. [Google Scholar] [CrossRef]
- Wang, L.; Sun, F.; Hao, F.; Qu, Z.; Gao, J.; Liu, M.; Qin, Y. A green trace K2CO3 induced catalytic activation strategy for developing coal-converted activated carbon as advanced candidate for CO2 adsorption and supercapacitors. Chem. Eng. J. 2020, 383, 123205. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Zhang, X.; Qin, H. Adsorption removal of methylene blue from aqueous solution by using bamboo charcoal. Fresenius Environ. Bull. 2009, 18, 369–376. [Google Scholar]
- Suhaimi, N.; Kooh, M.R.R.; Lim, C.M.; Chou Chao, C.-T.; Chou Chau, Y.-F.; Mahadi, A.H.; Chiang, H.-P. The use of gigantochloa bamboo-derived biochar for the removal of methylene blue from aqueous solution. Adsorp. Sci. Technol. 2022, 2022, 8245797. [Google Scholar] [CrossRef]
- Ismail, I.S.; Rashidi, N.A.; Yusup, S. Production and characterization of bamboo-based activated carbon through single-step H3PO4 activation for CO2 capture. Environ. Sci. Pollut. Res. 2022, 29, 12434–12440. [Google Scholar] [CrossRef]
- Sahu, U.K.; Ji, W.; Liang, Y.; Ma, H.; Pu, S. Mechanism enhanced active biochar support magnetic nano zero-valent iron for efficient removal of Cr (VI) from simulated polluted water. J. Environ. Chem. Eng. 2022, 10, 107077. [Google Scholar] [CrossRef]
- Bonyadi, Z.; Noghani, F.; Dehghan, A.; van der Hoek, J.P.; Giannakoudakis, D.A.; Ghadiri, S.K.; Anastopoulos, I.; Sarkhosh, M.; Colmenares, J.C.; Shams, M. Biomass-derived porous aminated graphitic nanosheets for removal of the pharmaceutical metronidazole: Optimization of physicochemical features and exploration of process mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125791. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Yang, Y.; Shang, Y.; Zhuang, X.; Li, H.; Zhou, Z. Combined process of visible light irradiation photocatalysis-coagulation enhances natural organic matter removal: Optimization of influencing factors and mechanism. Chem. Eng. J. 2019, 374, 48–759. [Google Scholar] [CrossRef]
- Jawad, A.H.; Sahu, U.K.; Jani, N.A.; ALOthman, Z.A.; Wilson, L.D. Magnetic crosslinked chitosan-tripolyphosphate/MgO/Fe3O4 nanocomposite for reactive blue 19 dye removal: Optimization using desirability function approach. Surf. Interfaces 2022, 28, 101698. [Google Scholar] [CrossRef]
- Sahu, U.K.; Sahu, S.; Mahapatra, S.S.; Patel, R.K. Synthesis and characterization of magnetic bio-adsorbent developed from Aegle marmelos leaves for removal of As(V) from aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Dalvand, A.; Nabizadeh, R.; Ganjali, M.R.M.; Nazmara, S.; Mahvi, A.H. Modeling of reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J. Magn. Magn. Mater. 2016, 404, 179–189. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A. Utilization of prepared activated biochar from water lily (Nymphaea lotus) stem for adsorption of malachite green dye from aqueous solution. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Jabar, J.M.; Owokotomo, I.A.; Ayinde, Y.T.; Alafabusuyi, A.M.; Olagunju, G.O.; Mobolaji, V.O. Characterization of prepared eco-friendly biochar from almond (Terminalia catappa L) leaf for sequestration of bromophenol blue (BPB) from aqueous solution. Carbon Lett. 2021. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A. Removal of cibacron blue 3G-A (CB) dye from aqueous solution using chemo-physically activated biochar from oil palm empty fruit bunch fiber. Arab. J. Chem. 2020, 13, 5417–5429. [Google Scholar] [CrossRef]
- Genli, N.; Kutluay, S.; Baytar, O.; Şahin, Ö. Preparation and characterization of activated carbon from hydrochar by hydrothermal carbonization of chickpea stem: An application in methylene blue removal by rsm optimization. Int. J. Phytoremediation 2022, 24, 88–100. [Google Scholar] [CrossRef]
- Gohr, M.S.; Abd-Elhamid, A.; El-Shanshory, A.A.; Soliman, H.M. Adsorption of cationic dyes onto chemically modified activated carbon: Kinetics and thermodynamic study. J. Mol. Liq. 2022, 346, 118227. [Google Scholar] [CrossRef]
- Spagnoli, A.A.; Giannakoudakis, D.A.; Bashkova, S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq. 2017, 229, 465–471. [Google Scholar] [CrossRef]
- Yusop, M.F.M.; Ahmad, M.A.; Rosli, N.A.; Abd Manaf, M.E. Adsorption of cationic methylene blue dye using microwave-assisted activated carbon derived from acacia wood: Optimization and batch studies. Arab. J. Chem. 2021, 14, 103122. [Google Scholar] [CrossRef]
- Sahu, U.K.; Mahapatra, S.S.; Patel, R.K. Application of box–behnken design in response surface methodology for adsorptive removal of arsenic from aqueous solution using CeO2/Fe2O3/graphene nanocomposite. Mater. Chem. Phys. 2018, 207, 233–242. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Wilson, L.D.; Syed-Hassan, S.S.A.; ALOthman, Z.A.; Khan, M.R. High surface area and mesoporous activated carbon from KOH-activated dragon fruit peels for methylene blue dye adsorption: Optimization and mechanism study. Chin. J. Chem. Eng. 2021, 32, 281–290. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Yu, X.; Mkandawire, V.; Ma, J.; Li, X. Removal of Fe2+ and Mn2+ from Polluted Groundwater by Insoluble Humic Acid/Tourmaline Composite Particles. Materials 2022, 15, 3130. [Google Scholar] [CrossRef] [PubMed]
- Husien, S.; El-taweel, R.M.; Salim, A.I.; Fahim, I.S.; Said, L.A.; Radwan, A.G. Review of activated carbon adsorbent material for textile dyes removal: Preparation, and modelling. Curr. Res. Green Sustain. Chem. 2022, 5, 100325. [Google Scholar] [CrossRef]
- Wang, X.; Fan, X.; Xie, H.; Li, X.; Hao, C. Polyacrylic acid/carboxymethyl cellulose/activated carbon composite hydrogel for removal of heavy metal ion and cationic dye. Cellulose 2022, 29, 483–501. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Freundlich’s adsorption isotherm. Phys. Chem. 1906, 57, 384. [Google Scholar]
- Temkin, M.I. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Porous structure and adsorptive properties of pineapple peel based activated carbons prepared via microwave assisted KOH and K2CO3 activation. Microporous Mesoporous Mater. 2012, 148, 191–195. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Firdaus Hum, N.N.M.; Rangabhashiyam, S.; Jawad, A.H.; Wilson, L.D.; Yaseen, Z.M.; Al-Kahtani, A.A.; Alothman, Z.A. Statistical modeling and mechanistic pathway for methylene blue dye removal by high surface area and mesoporous grass-based activated carbon using K2CO3 activator. J. Environ. Chem. Eng. 2021, 9, 105530. [Google Scholar] [CrossRef]
- Santana, G.M.; Trugilho, P.F.; Borges, W.M.; Bianchi, M.L.; Paes, J.B.; Nobre, J.R.C.; Morais, R.M. Activated carbon from bamboo (Bambusa vulgaris) waste using CO2 as activating agent for adsorption of methylene blue and phenol. Ciência Florest. 2019, 29, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Jabar, J.M.; Odusote, Y.A.; Ayinde, Y.T.; Yılmaz, M. African almond (Terminalia catappa L) leaves biochar prepared through pyrolysis using H3PO4 as chemical activator for sequestration of methylene blue dye. Results Eng. 2022, 14, 100385. [Google Scholar] [CrossRef]
- Dao, T.M.; Le Luu, T. Synthesis of activated carbon from macadamia nutshells activated by H2SO4 and K2CO3 for methylene blue removal in water. Bioresour. Technol. Rep. 2020, 12, 100583. [Google Scholar] [CrossRef]
- Jawad, A.H.; Sabar, S.; Ishak, M.A.M.; Wilson, L.D.; Ahmad Norrahma, S.S.; Talari, M.K.; Farhan, A.M. Microwave-assisted preparation of mesoporous-activated carbon from coconut (Cocos nucifera) leaf by H3PO4 activation for methylene blue adsorption. Chem. Eng. Commun. 2017, 204, 1143–1156. [Google Scholar] [CrossRef]
- Dakhil, I.H.; Ali, A.H. Adsorption of methylene blue dye from industrial wastewater using activated carbon prepared from agriculture wastes. Desalination Water Treat. 2021, 216, 372–378. [Google Scholar] [CrossRef]
- Baytar, O.; Ceyhan, A.A.; Şahin, Ö. Production of activated carbon from elaeagnus angustifolia seeds using H3PO4 activator and methylene blue and malachite green adsorption. Int. J. Phytoremediation 2021, 23, 693–703. [Google Scholar] [CrossRef]
- Mashhadi, S.; Javadian, H.; Ghasemi, M.; Saleh, T.A.; Gupta, V.K. Microwave-induced H2SO4 activation of activated carbon derived from rice agricultural wastes for sorption of methylene blue from aqueous solution. Desalin. Water Treat. 2016, 57, 21091–21104. [Google Scholar]
- Özhan, A.; Şahin, Ö.; Küçük, M.M.; Saka, C. Preparation and characterization of activated carbon from pine cone by microwave-induced ZnCl2 activation and its effects on the adsorption of methylene blue. Cellulose 2014, 21, 2457–2467. [Google Scholar] [CrossRef]
- El-Sayed, G.O.; Yehia, M.M.; Asaad, A.A. Assessment of activated carbon prepared from corncob by chemical activation with phosphoric acid. Water Resour. Ind. 2014, 7, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.K.; Prasad, R.; Shankar, R.; Khare, P.; Yadav, M. Adsorptive removal of methylene blue dye from soapnut shell & pineapple waste derived activated carbon. Int. J. Eng. Sci. Technol. 2021, 13, 81–87. [Google Scholar]
- Sahu, S.; Pahi, S.; Sahu, J.K.; Sahu, U.K.; Patel, R.K. Kendu (Diospyros melanoxylon roxb) fruit peel activated carbon—An efficient bioadsorbent for methylene blue dye: Equilibrium, kinetic, and thermodynamic study. Environ. Sci. Pollut. Res. Int. 2020, 27, 22579–22592. [Google Scholar] [CrossRef] [PubMed]










| Variables | Codes | Level 1 (−1) | Level 2 (0) | Level 3 (+1) |
|---|---|---|---|---|
| Dose (g/100 mL) | A | 0.02 | 0.05 | 0.08 |
| pH | B | 4 | 7 | 10 |
| Time (min) | C | 2 | 5 | 8 |
| Sample | Average Pore Size (nm) | BET Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|---|
| BWAC | 2.91 | 107.148 | 0.049 |
| Run | A: Dose (g) | B: pH | C: Time (min) | MB Removal (%) |
|---|---|---|---|---|
| 1 | 0.02 | 4 | 5 | 49 |
| 2 | 0.08 | 4 | 5 | 73 |
| 3 | 0.02 | 10 | 5 | 63 |
| 4 | 0.08 | 10 | 5 | 73 |
| 5 | 0.02 | 7 | 2 | 61 |
| 6 | 0.08 | 7 | 2 | 65 |
| 7 | 0.02 | 7 | 8 | 61 |
| 8 | 0.08 | 7 | 8 | 87 |
| 9 | 0.05 | 4 | 2 | 51 |
| 10 | 0.05 | 10 | 2 | 63 |
| 11 | 0.05 | 4 | 8 | 72 |
| 12 | 0.05 | 10 | 8 | 65 |
| 13 | 0.05 | 7 | 5 | 61 |
| 14 | 0.05 | 7 | 5 | 62 |
| 15 | 0.05 | 7 | 5 | 63 |
| 16 | 0.05 | 7 | 5 | 63 |
| 17 | 0.05 | 7 | 5 | 58 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 1192.31 | 9 | 132.48 | 31.49 | <0.0001 | Significant |
| A-Dose | 512.00 | 1 | 512.00 | 121.70 | <0.0001 | * |
| B-pH | 45.13 | 1 | 45.13 | 10.73 | 0.0136 | * |
| C-Time | 253.13 | 1 | 253.13 | 60.17 | 0.0001 | * |
| AB | 49.00 | 1 | 49.00 | 11.65 | 0.0112 | * |
| AC | 121.00 | 1 | 121.00 | 28.76 | 0.0010 | * |
| BC | 90.25 | 1 | 90.25 | 21.45 | 0.0024 | * |
| A2 | 82.44 | 1 | 82.44 | 19.60 | 0.0031 | * |
| B2 | 7.39 | 1 | 7.39 | 1.76 | 0.2266 | # |
| C2 | 30.13 | 1 | 30.13 | 7.16 | 0.0317 | * |
| Residual | 29.45 | 7 | 4.21 | |||
| Lack of Fit | 12.25 | 3 | 4.08 | 0.9496 | 0.4967 | # |
| Pure Error | 17.20 | 4 | 4.30 | |||
| Cor Total | 1221.76 | 16 |
| Process Parameters | Optimized Values (Predicted by Disability Function) | Confirmation Values (Experimental) |
|---|---|---|
| BWAC Dose (g) | 0.08 | 0.08 |
| pH | 7.61 | 7.62 |
| Time (min) MB removal (%) | 7.99 86.37 | 8 83.5 ± 3 |
| PSO | PFO | qe exp. (mg/g) | Concentration (mg/L) | ||||
|---|---|---|---|---|---|---|---|
| R2 | k2 × 102 (g/mg min) | qe cal (mg/g) | R2 | k1 (1/min) | qe cal (mg/g) | ||
| 0.99 | 5.107 | 24.89 | 0.99 | 0.62 | 23.51 | 23.83 | 20 |
| 0.96 | 1.412 | 53.54 | 0.90 | 0.46 | 50.07 | 55.29 | 40 |
| 0.97 | 0.289 | 76.83 | 0.93 | 0.17 | 72.21 | 78.55 | 60 |
| 0.94 | 0.263 | 84.18 | 0.86 | 0.13 | 83.64 | 83.01 | 80 |
| 0.93 | 0.088 | 94.75 | 0.85 | 0.07 | 81.25 | 107.5 | 100 |
| Model | Parameter | Values |
|---|---|---|
| Langmuir | qmax (mg/g) | 85.6 |
| Ka (L/mg) | 5.74 | |
| R2 | 0.95 | |
| Freundlich | KF (mg/g (L/mg)1/n) | 57.5 |
| N | 8.33 | |
| R2 | 0.84 | |
| Temkin | KT (L/mg) | 816.1 |
| bT (J/mol) | 282.8 | |
| R2 | 0.87 |
| Initial Material | Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| Pineapple peel waste Grass waste Bamboo African almond leaves Nutshells Coconut leaves | 462.10 364 298.82 263.95 261 250 | [49] [50] [51] [52] [53] [54] |
| Agriculture wastes | 148.8 | [55] |
| Bamboo waste | 85.6 | This Study |
| Elaeagnus Angustifolia seeds Rice agricultural waste Pine cone | 72 62.5 60.97 | [56] [57] [58] |
| Corncob | 28.65 | [59] |
| Pineapple waste | 9.61 | [60] |
| T (K) | Ln Kd | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (kJ/mol K) |
|---|---|---|---|---|
| 298.15 308.15 318.15 328.15 | 2.05 2.32 3.39 3.66 | −5.08 −5.94 −8.96 −9.98 | 35.67 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azlan Zahari, K.F.; Sahu, U.K.; Khadiran, T.; Surip, S.N.; ALOthman, Z.A.; Jawad, A.H. Mesoporous Activated Carbon from Bamboo Waste via Microwave-Assisted K2CO3 Activation: Adsorption Optimization and Mechanism for Methylene Blue Dye. Separations 2022, 9, 390. https://doi.org/10.3390/separations9120390
Azlan Zahari KF, Sahu UK, Khadiran T, Surip SN, ALOthman ZA, Jawad AH. Mesoporous Activated Carbon from Bamboo Waste via Microwave-Assisted K2CO3 Activation: Adsorption Optimization and Mechanism for Methylene Blue Dye. Separations. 2022; 9(12):390. https://doi.org/10.3390/separations9120390
Chicago/Turabian StyleAzlan Zahari, Khaizuran Fyrdaus, Uttam Kumar Sahu, Tumirah Khadiran, Siti Norasmah Surip, Zeid A. ALOthman, and Ali H. Jawad. 2022. "Mesoporous Activated Carbon from Bamboo Waste via Microwave-Assisted K2CO3 Activation: Adsorption Optimization and Mechanism for Methylene Blue Dye" Separations 9, no. 12: 390. https://doi.org/10.3390/separations9120390