Application of Response Surface Method for Determination of Optimized Conditions for Quantification of 1,4-Dioxane in Model System of Polyethylene Glycol 200
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Standard Solutions for Standard Curve
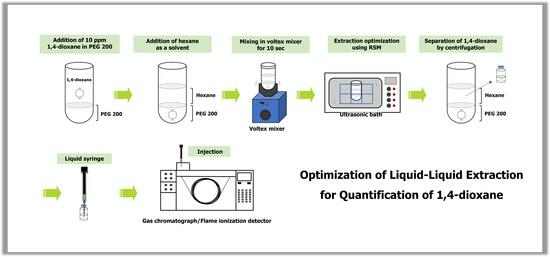
2.3. Liquid–Liquid Extraction of 1,4-Dioxane for Standard Solutions and Samples
2.4. Extraction Condition of 1,4-Dioxane in Ultrasonicfication
2.5. Experimental Design for Optimization of LLE Using RSM
2.6. Liquid–Liquid Extraction of 1,4-Dioxane for RSM
2.7. GC-FID Conditions
2.8. Statistical Analysis
3. Results and Discussion
3.1. Content of 1,4-Dioxane by Standard Curve in PEG 200
3.2. Effect of Ultrasound with Extraction Temperature and Time on Recovery Rate of 1,4-Dioxane
3.3. Optimization of Extraction Yield of 1,4-Dioxane Using Response Surface Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duncan, B.; Vavricka, E.; Morrison, R. A forensic overview of 1,4-dioxane. Environ. Claims J. 2004, 16, 69–79. [Google Scholar] [CrossRef]
- Pollitt, K.J.G.; Kim, J.-H.; Peccia, J.; Elimelech, M.; Zhang, Y.; Charkoftaki, G.; Hodges, B.; Zucker, I.; Huang, H.; Deziel, N.C. 1,4-Dioxane as an emerging water contaminant: State of the science and evaluation of research needs. Sci. Total Environ. 2019, 690, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.K.; Kappleman, W.B. Review of the Human Health Toxicology and Ecotoxicology of 1,4-Dioxane, Environmental Investigation and Remediation; CRC Press: Boca Raton, FL, USA, 2020; pp. 255–290. [Google Scholar]
- Eigenbrodt, C.; Rooney, E. Adsorption of 1,4–Dioxane on Activated Carbon with Regeneration by Titanium Dioxide/Ultraviolet Light; WPI: Worcester, MA, USA, 2014; p. 32. [Google Scholar]
- Dawson, D.; Fisher, H.; Noble, A.E.; Meng, Q.; Doherty, A.C.; Sakano, Y.; Vallero, D.; Tornero-Velez, R.; Cohen Hubal, E.A. Assessment of non-occupational 1,4-dioxane exposure pathways from drinking water and product use. Environ. Sci. Technol. 2022, 56, 5266–5275. [Google Scholar] [CrossRef]
- Wang, Y. Breakthrough in 2D-CSIA technology for 1,4-dioxane. Remediat. J. 2016, 27, 61–70. [Google Scholar] [CrossRef]
- Pundlik, M.; Sitharaman, B.; Kaur, I. Gas chromatographic determination of 1,4-dioxane at low parts-per-million levels in glycols. J. Chromatogr. Sci. 2001, 39, 73–76. [Google Scholar] [CrossRef]
- Lavilla, I.; Cabaleiro, N.; Bendicho, C. Main chemical contaminants in cosmetics: Regulatory aspects and analytical methods. In Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 331–383. [Google Scholar]
- Li, F.; Deng, D.; Li, M. Distinct catalytic behaviors between two 1,4-dioxane-degrading monooxygenases: Kinetics, inhibition, and substrate range. Environ. Sci. Technol. 2019, 54, 1898–1908. [Google Scholar] [CrossRef]
- Mohr, T.K. 1,4-Dioxane Chemistry, Uses, and Occurrence, Environmental Investigation and Remediation; CRC Press: Boca Raton, FL, USA, 2020; pp. 77–124. [Google Scholar]
- Chiang, S.Y.; Anderson, R.; Wilken, M.; Walecka-Hutchison, C. Practical perspectives of 1,4-dioxane investigation and remediation. Remediat. J. 2016, 27, 7–27. [Google Scholar] [CrossRef]
- Halden, R.U. Epistemology of contaminants of emerging concern and literature meta-analysis. J. Hazard. Mater. 2015, 282, 2–9. [Google Scholar] [CrossRef]
- Stepien, D.K.; Diehl, P.; Helm, J.; Thoms, A.; Püttmann, W. Fate of 1,4-dioxane in the aquatic environment: From sewage to drinking water. Water Res. 2014, 48, 406–419. [Google Scholar] [CrossRef]
- Adamson, D.T.; Uhlir, G.; Rauch, S.R.; Klein, T.; Danko, A.S. Trends in 1,4-dioxane analyses: Implications for identification and characterization of contaminated groundwater sites. Groundw. Monit. Remediat. 2021, 41, 29–40. [Google Scholar] [CrossRef]
- Polsky, C.; Schwarzman, M. The hidden success of a conspicuous law: Proposition 65 and the reduction of toxic chemical exposures. Ecol. LQ 2020, 47, 823. [Google Scholar]
- Broughton, A.; Sepulveda, A.; Foster, K.; Kruk, T.; Nickelsen, M.G.; Gillan, M.; Mohr, T.K. 1,4-Dioxane: Emerging technologies for an emerging contaminant. Remediat. J. 2019, 29, 49–63. [Google Scholar] [CrossRef]
- Zhang, S.; Gedalanga, P.B.; Mahendra, S. Advances in bioremediation of 1,4-dioxane-contaminated waters. J. Environ. Manag. 2017, 204, 765–774. [Google Scholar] [CrossRef]
- Guo, W.; Brodowsky, H. Determination of the trace 1,4-dioxane. Microchem. J. 2000, 64, 173–179. [Google Scholar] [CrossRef]
- Jones-Lepp, T.L. Emerging Contaminants in the Environment. Comprehensive Environmental Mass Spectrometry; U.S. Environmental Protection Agency: Las Vegas, NV, USA, 2012; p. 287.
- Kasiri, M.; Aleboyeh, H.; Aleboyeh, A. Modeling and optimization of heterogeneous photo-fenton process with response surface methodology and artificial neural networks. Environ. Sci. Technol. 2008, 42, 7970–7975. [Google Scholar] [CrossRef] [PubMed]
- Arslan-Alaton, I.; Tureli, G.; Olmez-Hanci, T. Treatment of azo dye production wastewaters using photo-fenton-like advanced oxidation processes: Optimization by response surface methodology. J. Photochem. Photobiol. A Chem. 2009, 202, 142–153. [Google Scholar] [CrossRef]
- Tanchotikul, U.; Hsieh, T.C.Y. Methodology for quantification of geosmin and levels in rangia clam (Rangia cuneata). J. Food Sci. 1990, 55, 1233–1235. [Google Scholar] [CrossRef]
- Son, H.-S.; Choi, S.-B.; Khan, E.; Zoh, K.-D. Removal of 1,4-dioxane from water using sonication: Effect of adding oxidants on the degradation kinetics. Water Res. 2006, 40, 692–698. [Google Scholar] [CrossRef]
- Alsohaimi, I.H.; Khan, M.R.; Ali, H.M.; Azam, M.; Alammari, A.M. Solvent extraction and gas chromatography–mass spectrometric determination of probable carcinogen 1,4-dioxane in cosmetic products. Sci. Rep. 2020, 10, 5214. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Pyo, H.S.; Park, S.J.; Park, S.K. Development of the analytical method for 1,4-dioxane in water by liquid–liquid extraction. Anal. Chim. Acta 2005, 548, 109–115. [Google Scholar] [CrossRef]
- Zhou, W. The determination of 1,4-dioxane in cosmetic products by gas chromatography with tandem mass spectrometry. J. Chromatogr. A 2019, 1607, 460400. [Google Scholar] [CrossRef]



| Category | Coded and Experimental Values of Independent Variables | ||||
|---|---|---|---|---|---|
| Coded Value | −1 | 0 | +1 | ||
| A | Experimental parameters | Sample (g) | 3 | 5 | 7 |
| B | Solvent (mL) | 3 | 5 | 7 | |
| C | Centrifugation speed (rpm) | 5000 | 10,000 | 15,000 | |
| Run | Coded and Experimental Values of Independent Variables | Response | ||
|---|---|---|---|---|
| A: Sample Amount (g) | B: Solvent Volume (mL) | C: Centrifugation Speed (rpm) | Y: Yield (%) | |
| 1 | +1 (7) | 0 (5) | −1 (5000) | 75.1 ± 8.1 |
| 2 | +1 (7) | −1 (3) | 0 (10,000) | 86.6 ± 1.5 |
| 3 | −1 (3) | 0 (5) | −1 (5000) | 42.4 ± 3.2 |
| 4 | +1 (7) | 0 (5) | +1 (15,000) | 77.7 ± 5.4 |
| 5 | 0 (5) | 0 (5) | 0 (10,000) | 56.4 ± 8.3 |
| 6 | −1 (3) | −1 (3) | 0 (10,000) | 47.7 ± 4.7 |
| 7 | 0 (5) | 0 (5) | 0 (10,000) | 56.7 ± 2.2 |
| 8 | −1 (3) | 0 (5) | +1 (15,000) | 52.5 ± 2.2 |
| 9 | 0 (5) | −1 (3) | −1 (5000) | 64.7 ± 4.7 |
| 10 | −1 (3) | +1 (7) | 0 (10,000) | 43.3 ± 5.7 |
| 11 | 0 (5) | −1 (3) | +1 (15,000) | 68.2 ± 6.4 |
| 12 | 0 (5) | +1 (7) | −1 (5000) | 45.0 ± 5.8 |
| 13 | 0 (5) | 0 (5) | 0 (10,000) | 57.6 ± 5.8 |
| 14 | +1 (7) | +1 (7) | 0 (10,000) | 55.3 ± 9.8 |
| 15 | 0 (5) | +1 (7) | +1 (15,000) | 53.6 ± 4.2 |
| 16 | 0 (5) | 0 (5) | 0 (10,000) | 59.0 ± 2.2 |
| 17 | 0 (5) | 0 (5) | 0 (10,000) | 59.5 ± 1.8 |
| Source | df | Estimated Value | p-Value |
|---|---|---|---|
| Prob > F | |||
| Intercept | 1 | +4.36000 | <0.0001 *** |
| A-Weight of sample | 1 | +11.53750 | <0.0001 *** |
| B-Volume of solvent | 1 | +7.33750 | <0.0001 *** |
| C-Centrifugation | 1 | −0.000574 | 0.0004 ** |
| AB | 1 | −1.68125 | <0.0001 *** |
| AC | 1 | −0.000187 | 0.0326 * |
| BC | 1 | +0.000128 | 0.1138 NS |
| A2 | 1 | +0.554375 | 0.0146 * |
| B2 | 1 | −0.458125 | 0.0323 * |
| C2 | 1 | +7.47000 × 10−8 | 0.0300 * |
| Residual | 7 | ||
| Lack of Fit | 3 | 0.4354 | |
| Pure Error | 4 | ||
| Corrected Total | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-C.; Park, S.-Y.; Kim, H.-R.; Cho, Y.-S.; Magollah, T.M.; Mok, J.H.; Lee, Y.-B. Application of Response Surface Method for Determination of Optimized Conditions for Quantification of 1,4-Dioxane in Model System of Polyethylene Glycol 200. Separations 2023, 10, 495. https://doi.org/10.3390/separations10090495
Kim M-C, Park S-Y, Kim H-R, Cho Y-S, Magollah TM, Mok JH, Lee Y-B. Application of Response Surface Method for Determination of Optimized Conditions for Quantification of 1,4-Dioxane in Model System of Polyethylene Glycol 200. Separations. 2023; 10(9):495. https://doi.org/10.3390/separations10090495
Chicago/Turabian StyleKim, Myung-Chan, Su-Yeon Park, Hyo-Rim Kim, Yun-Sung Cho, Tabu Mungia Magollah, Jin Hong Mok, and Yang-Bong Lee. 2023. "Application of Response Surface Method for Determination of Optimized Conditions for Quantification of 1,4-Dioxane in Model System of Polyethylene Glycol 200" Separations 10, no. 9: 495. https://doi.org/10.3390/separations10090495