Kinetics of Ni and Co Recovery via Oxygen-Enriched Pressure Leaching from Waste Lithium-Ion Batteries
Abstract
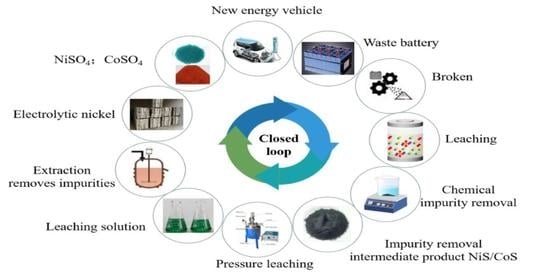
:1. Introduction
2. Experimental Section
2.1. Materials and Equipment
2.2. Experimental Methods
2.3. Process Flow Chart
2.4. Analysis Method
- (1)
- Moisture analysis method
- (2)
- Metal content analysis method
2.5. Evaluation Indicators
3. Results and Discussion
3.1. Effect of the Initial Concentration of Sulfuric Acid on the Leaching Rates of Ni and Co
3.2. Effect of Stirring Speed on the Leaching Rates of Ni and Co
3.3. Effect of Reaction Temperature on the Leaching Rates of Ni and Co
3.4. Effect of Leaching Time on the Leaching Rates of Ni and Co
3.5. Reproducible Experimental Study
3.6. Analysis of the Effect of Oxygen Pressure on Acid Leaching of the Mixture of NiS and CoS
3.6.1. Oxidation Reaction of Sulfur
3.6.2. Oxidation Leaching Mechanism for NiS and CoS
3.6.3. Oxidation of H2S
3.6.4. Catalytic Oxidation of Fe Ions
3.7. Leaching Kinetics Model
3.8. Apparent Activation Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Liu, G.; Wang, F.; Liu, Y. Market analysis of waste power battery recycling in 2020. Compr. Util. Resour. China 2020, 38, 102–105. [Google Scholar] [CrossRef]
- Astuti, W.; Hirajima, T.; Sasaki, K.; Okibe, N. Kinetics of Nickel Extraction from Indonesian Saprolitic Ore by Citric Acid Leaching under Atmospheric Pressure. Min. Metall Explor. 2015, 32, 176–185. [Google Scholar] [CrossRef]
- Thubakgale, C.K.; Mbaya, R.K.K.; Shongwe, M.B. Characteristics of leaching of nickel from a mafic overburden in sulfuric acid and sodium chloride medium at atmospheric pressure. JOM 2019, 71, 4616–4623. [Google Scholar] [CrossRef]
- Luo, W.; Feng, Q.; Ou, L.; Zhang, G.F.; Chen, Y. Kinetics of Saprolitic Laterite Leaching by Sulphuric Acid at Atmospheric Pressure. Miner. Eng. 2010, 23, 458–462. [Google Scholar] [CrossRef]
- McDonald, R.G.; Whittington, B.I. Atmospheric acid leaching of nickel laterites review: Part I. Sulphuric acid technologies. Hydrometallurgy 2008, 91, 35–55. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Kaya, M. Atmospheric pressure acid leaching of Caldag lateritic nickel ore. Int. J. Miner. Process. 2016, 150, 1–8. [Google Scholar] [CrossRef]
- Swamy, Y.V.; Rao, K.V.K. Extraction of Nickel and Cobalt from Reduced Chromite Overburden by Dilute Sulfuric-Acid Leaching. Trans. Indian Inst. Metals 1994, 47, 409. [Google Scholar]
- Whittington, B.; Muir, D. Pressure acid leaching of nickel laterites: A review. Miner. Process. Extr. Metullargy Rev. 2000, 21, 527. [Google Scholar] [CrossRef]
- Weston, D. Hydrometallurgical Treatment of Nickel Group Ores. U.S. Patent 3793432, 19 February 1974. [Google Scholar]
- Weston, D. Hydrometallurgical Treatment of Nickel, Cobalt and Copper Containing Materials. U.S. Patent 3793430, 19 February 1974. [Google Scholar]
- Monhemius, A.J. Treatment of laterite ores of nickel to produce ferronickel, matte or precipitated sulphide. In Extractive Metallurgy of Nickel; John Wiley & Sons Ltd.: Chichester, UK, 1987; pp. 63–71. [Google Scholar]
- Xu, Y.; Xie, Y.; Yan, L.; Yang, R.D. A new method for recovering valuable metals from low-grade nickeliferous oxide ores. Hydrometallurgy 2005, 80, 280–285. [Google Scholar] [CrossRef]
- Wang, X.; McDonald, R.G.; Hart, R.D.; Li, J.; Riessen, A. Acid resistance of goethite in nickel laterite ore from Western Australia. Part II. Effect of liberating cementations on acid leaching performance. Hydrometallurgy 2014, 141, 49–58. [Google Scholar] [CrossRef]
- Kosyakov, A.; Hamalainen, M.; Gromov, P.; Kasikov, A.; Masloboev, V.; Neradovsky, Y. Autoclave processing of low grade copper-nickel concentrates. Hydrometallurgy 1995, 39, 223–234. [Google Scholar] [CrossRef]
- Huang, K.; Li, Q.; Chen, J. Recovery of copper, nickel and cobalt from acidic pressure leaching solutions of low-grade sulfide flotation concentrates. Miner. Eng. 2007, 20, 722–728. [Google Scholar] [CrossRef]
- Tong, L.; Dreisinger, D. Interfacial properties of liquid sulfur in the pressure leaching of nickel concentrate. Miner. Eng. 2009, 22, 456–461. [Google Scholar] [CrossRef]
- Li, Y.; Perederiy, I.; Papangelakis, V.G. Cleaning of waste smelter slags and recovery of valuable metals by pressure oxidative leaching. J. Hazard. Mater. 2008, 152, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Herreros, O.; Quiroz, R.; Manzano, E.; Boub, C.; Viñalsb, J. Copper extraction from reverberatory and flash furnace slags by chlorine leaching. Hydrometallurgy 1998, 49, 87–101. [Google Scholar] [CrossRef]
- Provis, J.L.; Deventer, J.S.J.; Rademan, J.A.M.; Lorenzen, L. A kinetic model for the acid-oxygen pressure leaching of Ni–Cu matte. Hydrometallurgy 2003, 70, 83–99. [Google Scholar] [CrossRef]
- Li, Y.; Papangelakis, V.G.; Perederiy, I. High pressure oxidative acid leaching of nickel smelter slag: Characterization of feed and residue. Hydrometallurgy 2009, 9, 185–193. [Google Scholar] [CrossRef]
- Loveday, B.K. The use of oxygen in high pressure acid leaching of nickel laterites. Miner. Eng. 2008, 21, 533–538. [Google Scholar] [CrossRef]
- Kar, B.B.; Swamy, Y.V.; Murthy, B.V.R. Design of experiments to study the extraction of nickel from lateritic ore by sulphatization using sulphuric acid. Hydrometallurgy 2000, 56, 387–394. [Google Scholar] [CrossRef]
- Rubisov, D.H.; Krowinkel, J.M.; Papangelakis, V.G. Sulphuric acid pressure leaching of laterites—Universal kinetics of nickel dissolution for limonites and limonitic/saprolitic blends. Hydrometallurgy 2000, 58, 1–11. [Google Scholar] [CrossRef]
- Whittington, B.I.; Johnson, J.A.; Quan, L.P.; McDonald, R.G.; Muiret, D.M. Pressure acid leaching of arid-region nickel laterite ore: Part II. Effect of ore type. Hydrometallurgy 2003, 70, 47–62. [Google Scholar] [CrossRef]
- Whittington, B.I.; McDonald, R.G.; Johnson, J.A.; Muir, D.M. Pressure acid leaching of arid-region nickel laterite ore: Part I: Effect of water quality. Hydrometallurgy 2003, 70, 31–46. [Google Scholar] [CrossRef]
- Huang, Y.; Song, L.; Liu, X.; Xiao, Y.F.; Wu, Y.; Chen, J.Y.; Wu, F.; Gu, Z.W. Hydroxyapatite coatings deposited by liquid precursor plasma spraying: Controlled dense and porous microstructures and osteoblastic cell responses. Biofabrication 2010, 2, 045003. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.X.; Wang, Y.L. Extraction and Refining of Precious Metals, Revised Edition; Central South University Press: Changsha, China, 2003; pp. 333–342. [Google Scholar]
- Homma, S.; Ogata, S.; Koga, J.; Matsumoto, S. Gas–solid reaction model for a shrinking spherical particle with unreacted shrinking core. Chem. Eng. J. 2005, 60, 4971–4980. [Google Scholar] [CrossRef]












| Element | Ni | Co | Fe | Al | Si | S | H2O |
|---|---|---|---|---|---|---|---|
| Content | 32.48 | 0.45 | 1.08 | <0.001 | <0.001 | 28.62 | 37.37 |
| Experiment Number | Leaching Rate (%) | Content in Leached Residue (%) | ||
|---|---|---|---|---|
| Ni | Co | Ni | Co | |
| 1 | 99.48 | 99.24 | 0.42 | 0.015 |
| 2 | 99.62 | 99.52 | 0.48 | 0.018 |
| 3 | 99.36 | 99.39 | 0.45 | 0.016 |
| Average | 99.49 | 99.38 | 0.45 | 0.016 |
| Extraction Temperature K | Rate Constant min−1 | Correlation Coefficient |
|---|---|---|
| 363 | 0.0007 | 0.95 |
| 373 | 0.0012 | 0.98 |
| 383 | 0.0017 | 0.98 |
| Extraction Temperature K | Rate Constant min−1 | Correlation Coefficient |
|---|---|---|
| 363 | 0.0007 | 0.96 |
| 373 | 0.00105 | 0.97 |
| 383 | 0.00115 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Shi, L.; Qu, T.; Yang, Z.; Lin, L.; Xie, G.; Xu, B. Kinetics of Ni and Co Recovery via Oxygen-Enriched Pressure Leaching from Waste Lithium-Ion Batteries. Separations 2023, 10, 64. https://doi.org/10.3390/separations10020064
Peng X, Shi L, Qu T, Yang Z, Lin L, Xie G, Xu B. Kinetics of Ni and Co Recovery via Oxygen-Enriched Pressure Leaching from Waste Lithium-Ion Batteries. Separations. 2023; 10(2):64. https://doi.org/10.3390/separations10020064
Chicago/Turabian StylePeng, Xuebin, Lei Shi, Tao Qu, Zheng Yang, Lin Lin, Gang Xie, and Baoqiang Xu. 2023. "Kinetics of Ni and Co Recovery via Oxygen-Enriched Pressure Leaching from Waste Lithium-Ion Batteries" Separations 10, no. 2: 64. https://doi.org/10.3390/separations10020064