Thermal Degradation of Cassava Rhizome in Thermosyphon-Fixed Bed Torrefaction Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
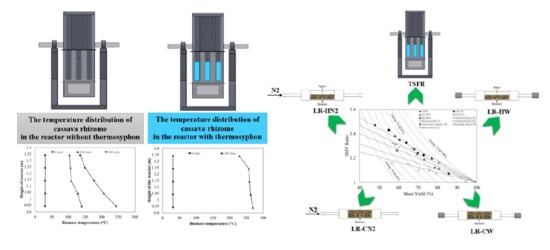
2.2. Thermosyphon - Fixed Bed Torrefaction Reactor
2.3. Laboratory fixed Bed Torrefaction Reactor
2.4. Experimental Procedure
2.4.1. Temperature Distribution of Cassava Rhizome and Heating Rate
2.4.2. Torrefied Char Preparation and Properties Analysis
3. Results and Discussion
3.1. Temperature Distribution of Cassava Rhizome and Heating Rate
3.2. Comparison of Torrefied Char Properties
3.3. Energy Yield
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of torrefaction process parameters on biomass feedstock upgrading. Fuel 2012, 91, 147–154. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.-J.; Kuang, J.-H.; Zhang, Y.-L.; Luo, Y.-H. Pretreatment of agricultural residues for co-gasification via torrefaction. J. Anal. Appl. Pyrolysis 2009, 86, 331–337. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Shield, I.; Williams, P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–866. [Google Scholar] [CrossRef]
- Pimchuai, A.; Dutta, A.; Basu, P. Torrefaction of agriculture residue to enhance combustible properties. Energy Fuels 2010, 24, 4638–4645. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Felfli, F.F.; Luengo, C.A.; Suárez, J.A.; Beaton, P.A. Wood briquette torrefaction. Energy Sustain. Dev. 2005, 9, 19–22. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Legros, R.; Bi, X.T.; Lim, C.J.; Sokhansanj, S. Torrefaction of sawdust in a fluidized bed reactor. Bioresour. Technol. 2012, 103, 453–458. [Google Scholar] [CrossRef]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of Temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrolysis 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Sabil, K.M.; Aziz, M.A.; Lal, B.; Uemura, Y. Effects of torrefaction on the physiochemical properties of oil palm empty fruit bunches mesocarp fiber and kernel shell. Biomass Bioenergy 2013, 56, 351–360. [Google Scholar] [CrossRef]
- Phanphanich, M.; Mani, S. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood: Part 2. Analysis of products. J. Anal. Appl. Pyrolysis 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, T.G.; Jone, J.M.; Williams, A.; Waldron, D.J. An investigation of the grindability of two torrefied energy crops. Fuel 2010, 89, 3911–3918. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-H.; Cheng, W.-Y.; Lu, K.-M.; Huang, Y.-P. An evaluation on improvement of pulverized biomass property for solid fuel through torrefaction. Appl. Energy 2011, 88, 3636–3644. [Google Scholar] [CrossRef]
- Chen, W.-H.; Huang, M.-Y.; Chang, J.-S.; Chen, C.Y. Thermal decomposition dynamics and severity of microalgae residues in torrefaction. Bioresour. Technol. 2014, 169, 258–264. [Google Scholar] [CrossRef]
- Guo, J.; Lua, A.C. Kinetic study on pyrolysis of extracted oil palm fiber: Isothermal and non-isothermal conditions. J. Therm. Anal. Calorim. 2000, 59, 763–774. [Google Scholar] [CrossRef]
- Guo, J.; Lua, A.C. Kinetic study on pyrolytic process of oil-palm solid waste using two-step consecutive reaction model. Biomass Bioenergy 2001, 20, 223–233. [Google Scholar] [CrossRef]
- Soponpongpipat, N.; Sae-Ueng, U. The effect of biomass bulk arrangements on the decomposition pathways in the torrefaction process. Renew. Energy 2015, 81, 679–684. [Google Scholar] [CrossRef]
- Dhungana, A.; Basu, P.; Dutta, A. Effects of Reactor Design on the Torrefaction of Biomass. J. Energy Resour. Technol. 2012, 134, 041801. [Google Scholar] [CrossRef]
- Department of Alternative Energy Development and Efficiency, Ministry of Energy. Available online: https://www.dede.go.th/ewt_news.php?nid=486 (accessed on 19 February 2020).
- Budde, K.P.; Megha, R.; Patel, R.; Pandey, J. Investigating effects of temperature on fuel properties of torrefied biomass for bio-energy systems. Energy Sources Part A 2018, 41, 1140–1148. [Google Scholar] [CrossRef]
- Mok, W.S.L.; Antal, M.J.; Szabo, P.; Varhegyi, G.; Zelei, B. Formation of charcoal from biomass in a sealed reactor. Ind. Eng. Chem. Res. 1992, 31, 1162–1166. [Google Scholar] [CrossRef]
- Wannapeera, J.; Worasuwannarak, N. Examinations of chemical properties and pyrolysis behaviors of torrefied woody biomass prepared at the same torrefaction mass yields. J. Anal. Appl. Pyrolysis 2015, 115, 279–287. [Google Scholar] [CrossRef]
- Ahiduzzaman, M.D.; Islam, A.K.M.S. Energy Yield of Torrefied Rice Husk at Atmospheric Condition. Procedia Eng. 2015, 105, 719–724. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Song, K. Thermal and chemical characteristics of torrefied biomass derived from a generated volatile atmosphere. Energy 2018, 165, 235–245. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Fonts, I.; Ábrego, J.; Ceamanos, J.; Gea, G. Sewage sludge torrefaction in a fluidized bed reactor. Chem. Eng. J. 2013, 222, 534–545. [Google Scholar] [CrossRef]
- Lin, Y.L. Effects of Microwave—Induced Torrefaction on Waste Straw Upgrading. Int. J. Chem. Eng. Appl. 2015, 6, 401–404. [Google Scholar] [CrossRef] [Green Version]











| Reactor Type | Heating Medium/Heat Source | Size of Heat Transfer Surface Area of Reactor | Difficulty to Operate the Process | Moving or Special Element | Limitation to Scale Up |
|---|---|---|---|---|---|
| Direct heating reactor | |||||
| 1. Fixed bed | Flue gas, Inert gas, or Superheated steam | N/A | Hard | Inert gas heat exchanger or superheated steam generator, Inert gas compressor | - Nonuniform heat distribution, when the thickness of the biomass bed is high - Difficulty to control the oxygen amount in flue gases - Price of inert gas - High construction cost for superheated steam generator |
| 2. Rotating drum | Flue gas or Superheated steam | N/A | Hard | Drum and driver set, superheated steam generator | - High construction cost for the superheated steam generator and the reactor - Difficulty to control the oxygen amount in the flue gases |
| 3. Fluidized bed | Solid medium and/or inert gas | N/A | Very hard | Gas/Air compressor, biomass/solid medium separator, | - Biomass/solid medium separation - High construction cost for peripheral equipment, such as the air/gas compressor and particle separator - Price of inert gas |
| 4. Moving bed | Flue gas or superheated steam | N/A | Hard | Conveyor belt | - High construction cost for the superheated steam generator and the reactor - Difficulty to control the oxygen amount in flue gases |
| 5. Microwave | Microwave | N/A | Hard | Microwave generator | - High construction cost for the reactor - High energy consumption |
| Indirect heating reactor | |||||
| 1. Fixed bed | Combustion or electrical heater | Large | Easy | No moving part | - Nonuniform heat distribution when the thickness of the biomass bed is high |
| 2. Screw | Combustion or electrical heater | Large | Medium | Screw and its driver set | - Nonuniform heat distribution when the thickness of the biomass bed is high |
| 3. Rotating drum | Combustion or electrical heater | Large | Medium | Drum and its driver set | - Nonuniform heat distribution when the thickness of the biomass bed is high |
| 4. New reactor design in present work | Combustion | Small | Easy | No moving part | - Uniform heat distribution when the thickness of the biomass bed is high, and low construction cost |
| Reactor | Torrefied Temperature (°C) | HHV (MJ/kg) | Mass Yield (%) | Energy Yield (%) |
|---|---|---|---|---|
| Raw cassava rhizome | - | 16.29 | 100 | 100 |
| Thermosyphon-fixed bed reactor (TSFR) | 230 | 20.24 | 69.89 | 86.86 |
| 260 | 21.99 | 60.43 | 81.60 | |
| 280 | 23.97 | 47.84 | 70.43 | |
| Laboratory reactor with compact bulk arrangement and without purge gas (LR-CW) | 230 | 19.96 | 72.89 | 89.34 |
| 260 | 21.58 | 63.96 | 84.76 | |
| 280 | 23.29 | 54.96 | 78.59 | |
| Laboratory reactor with hollow bulk arrangement and without purge gas (LR-HW) | 230 | 19.53 | 76.96 | 92.28 |
| 260 | 20.78 | 68.07 | 86.86 | |
| 280 | 22.52 | 58.08 | 80.32 | |
| Laboratory reactor with compact bulk arrangement and N2 purge gas (LR-CN2) | 230 | 18.93 | 80.58 | 93.67 |
| 260 | 20.05 | 69.38 | 85.41 | |
| 280 | 21.37 | 58.50 | 76.75 | |
| Laboratory reactor with hollow bulk arrangement and N2 purge gas (LR-HN2) | 230 | 18.16 | 80.62 | 89.91 |
| 260 | 19.35 | 73.15 | 86.92 | |
| 280 | 20.62 | 59.08 | 74.79 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soponpongpipat, N.; Nanetoe, S.; Comsawang, P. Thermal Degradation of Cassava Rhizome in Thermosyphon-Fixed Bed Torrefaction Reactor. Processes 2020, 8, 267. https://doi.org/10.3390/pr8030267
Soponpongpipat N, Nanetoe S, Comsawang P. Thermal Degradation of Cassava Rhizome in Thermosyphon-Fixed Bed Torrefaction Reactor. Processes. 2020; 8(3):267. https://doi.org/10.3390/pr8030267
Chicago/Turabian StyleSoponpongpipat, Nitipong, Suwat Nanetoe, and Paisan Comsawang. 2020. "Thermal Degradation of Cassava Rhizome in Thermosyphon-Fixed Bed Torrefaction Reactor" Processes 8, no. 3: 267. https://doi.org/10.3390/pr8030267