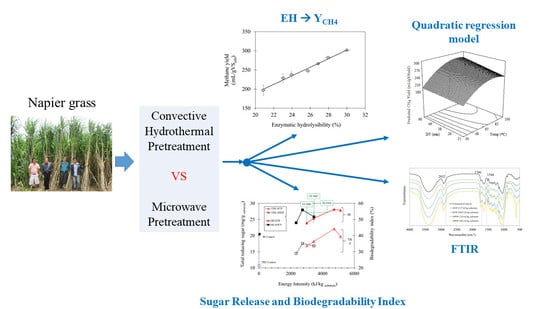
Comparing Low-Temperature Hydrothermal Pretreatments through Convective Heating versus Microwave Heating for Napier Grass Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Convective Hydrothermal Pretreatment (CHTP) of the Substrate
2.3. Enzymatic Hydrolysis Assay
2.4. Biochemical Methane Potential (BMP) Assay
2.5. Determination of Biomethanation Potential from Enzymatic Hydrolysibility, and Quadratic Prediction Model
2.6. Comparison between Convective and Microwave Hydrothermal Pretreatments of the Napier Grass Biomass
2.6.1. Microwave Pretreatment (MWP)
2.6.2. Energy Consumption Calculations
2.6.3. Biodegradability Index of Substrate
2.7. Analytical Procedures
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effects of Temperature and Detention Time of Convective Hydrothermal Pretreatment (CHTP) on Methane Yield
3.2. Enzymatic Hydrolysis and Correlation to Methane Yield
3.3. Modelling Methane Yield in Convective Hydrothermal Pretreatment
3.4. Comparison between Convective Hydrothermal and Microwave Pretreatments
3.4.1. Release of Sugars and Lignocellulose Derivatives into Liquid Hydrolysate
3.4.2. Difference in Biodegradability of the Pretreated Grass Biomass
3.4.3. Relative Energy Gain from Thermal Pretreatments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van De Ven, D.-J.; Fouquet, R. Historical energy price shocks and their changing effects on the economy. Energy Econ. 2017, 62, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Rizwan, M.; Shah, S.H.; Mujtaba, G.; Mahmood, Q.; Rashid, N.; Shah, F.A. Ecofuel feedstocks and their prospect. In Advanced Biofuels; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 3–16. [Google Scholar]
- Farrell, G.; Simons, S.A.; Hillocks, R.J. Pests, diseases and weeds of Napier grass, Pennisetum purpureum: A review. Int. J. Pest Manag. 2002, 48, 39–48. [Google Scholar] [CrossRef]
- Sawasdee, V.; Pisutpaisal, N. Feasibility of biogas production from Napier grass. Energy Procedia 2014, 61, 1229–1233. [Google Scholar] [CrossRef] [Green Version]
- Ngammuangtueng, P.; Jakrawatana, N.; Gheewala, S.H. Nexus Resources efficiency assessment and management towards transition to sustainable bioeconomy in Thailand. Resour. Conserv. Recycl. 2020, 160, 104945. [Google Scholar] [CrossRef]
- Li, H.; Pu, Y.; Kumar, R.; Ragauskas, A.J.; Wyman, C.E. Investigation of lignin deposition on cellulose during hydrothermal pretreatment, its effect on cellulose hydrolysis, and underlying mechanisms. Biotechnol. Bioeng. 2013, 111, 485–492. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Himmel, M.; Baker, J.O.; Overend, R. A review of: “Enzymatic conversion of biomass for fuels production”. Fuel Sci. Technol. Int. 1996, 14, 339. [Google Scholar] [CrossRef]
- Malherbe, S.; Cloete, T. Lignocellulose biodegradation: Fundamentals and applications. Rev. Environ. Sci. Bio/Technol. 2002, 1, 105–114. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M.R. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Deng, C.; Ding, L.; Bose, A.; Murphy, J.D. Improving gaseous biofuel production from seaweed Saccharina latissima: The effect of hydrothermal pretreatment on energy efficiency. Energy Convers. Manag. 2019, 196, 1385–1394. [Google Scholar] [CrossRef]
- Luo, T.; Huang, H.; Mei, Z.; Shen, F.; Ge, Y.; Hu, G.; Meng, X. Hydrothermal pretreatment of rice straw at relatively lower temperature to improve biogas production via anaerobic digestion. Chin. Chem. Lett. 2019, 30, 1219–1223. [Google Scholar] [CrossRef]
- Song, X.; Wachemo, A.C.; Zhang, L.; Bai, T.; Li, X.; Zuo, X.; Yuan, H. Effect of hydrothermal pretreatment severity on the pretreatment characteristics and anaerobic digestion performance of corn stover. Bioresour. Technol. 2019, 289, 121646. [Google Scholar] [CrossRef]
- Tekin, K.; Karagoz, S.; Bektaş, S. A review of hydrothermal biomass processing. Renew. Sustain. Energy Rev. 2014, 40, 673–687. [Google Scholar] [CrossRef]
- Fernández-Cegrí, V.; De La Rubia, M.A.; Raposo, F.; Borja, R. Effect of hydrothermal pretreatment of sunflower oil cake on biomethane potential focusing on fibre composition. Bioresour. Technol. 2012, 123, 424–429. [Google Scholar] [CrossRef]
- Rafique, R.; Poulsen, T.G.; Nizami, A.S.; Asam, Z.-U.-Z.; Murphy, J.D.; Kiely, G.; Nizami, A.S. Effect of thermal, chemical and thermo-chemical pre-treatments to enhance methane production. Energy 2010, 35, 4556–4561. [Google Scholar] [CrossRef]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef]
- Tan, C.; Saritpongteeraka, K.; Kungsanant, S.; Charnnok, B.; Chaiprapat, S. Low temperature hydrothermal treatment of palm fiber fuel for simultaneous potassium removal, enhanced oil recovery and biogas production. Fuel 2018, 234, 1055–1063. [Google Scholar] [CrossRef]
- Díaz, A.B.; Moretti, M.M.D.S.; Bezerra-Bussoli, C.; Nunes, C.D.C.C.; Blandino, A.; Da Silva, R.; Gomes, E. Evaluation of microwave-assisted pretreatment of lignocellulosic biomass immersed in alkaline glycerol for fermentable sugars production. Bioresour. Technol. 2015, 185, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, W.; Chiueh, P.; Kuan, W.; Lo, S. Microwave torrefaction of rice straw and pennisetum. Bioresour. Technol. 2012, 123, 1–7. [Google Scholar] [CrossRef]
- Marin, J.; Kennedy, K.J.; Eskicioglu, C. Effect of microwave irradiation on anaerobic degradability of model kitchen waste. Waste Manag. 2010, 30, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Krishna, B.B.; Kumar, J.; Bhaskar, T. Opportunities for utilization of non-conventional energy sources for biomass pretreatment. Bioresour. Technol. 2016, 199, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Pecorini, I.; Baldi, F.; Carnevale, E.; Corti, A. Biochemical methane potential tests of different autoclaved and microwaved lignocellulosic organic fractions of municipal solid waste. Waste Manag. 2016, 56, 143–150. [Google Scholar] [CrossRef]
- Sapci, Z. The effect of microwave pretreatment on biogas production from agricultural straws. Bioresour. Technol. 2013, 128, 487–494. [Google Scholar] [CrossRef]
- Jackowiak, D.; Frigon, J.; Ribeiro, T.; Pauss, A.; Guiot, S. Enhancing solubilisation and methane production kinetic of switchgrass by microwave pretreatment. Bioresour. Technol. 2011, 102, 3535–3540. [Google Scholar] [CrossRef] [Green Version]
- Pellera, F.-M.; Gidarakos, E. Microwave pretreatment of lignocellulosic agroindustrial waste for methane production. J. Environ. Chem. Eng. 2017, 5, 352–365. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, March 2008. [Google Scholar]
- Yu, X.; Liu, Y.; Cui, Y.; Cheng, Q.; Zhang, Z.; Lu, J.H.; Meng, Q.; Teng, L.; Ren, X. Measurement of filter paper activities of cellulase with microplate-based assay. Saudi J. Boil. Sci. 2015, 23, S93–S98. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.-Y.; Lee, J.-W. Hydrothermal Treatment. In Pretreatment of Biomass; Elsevier: Amsterdam, The Netherlands, 2015; pp. 61–74. [Google Scholar]
- Sugiharto, Y.E.C.; Harimawan, A.; Kresnowati, M.T.A.P.; Purwadi, R.; Mariyana, R.; Fitriana, H.N.; Hosen, H.F. Enzyme feeding strategies for better fed-batch enzymatic hydrolysis of empty fruit bunch. Bioresour. Technol. 2016, 207, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van ’T Riet, K. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Khanal, S.K. Bioenergy: Principles and Applications; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association New York: Washington, DC, USA, 2017. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, X.; Dai, R.; Wang, Y.; Ma, P. Effect of low temperature of thermal pretreatment on anaerobic digestion of textile dyeing sludge. Bioresour. Technol. 2017, 243, 426–432. [Google Scholar] [CrossRef]
- Prorot, A.; Julien, L.; Christophe, D.; Patrick, L. Sludge disintegration during heat treatment at low temperature: A better understanding of involved mechanisms with a multiparametric approach. Biochem. Eng. J. 2011, 54, 178–184. [Google Scholar] [CrossRef]
- Wang, D.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Luo, T.; Mei, Z. Can hydrothermal pretreatment improve anaerobic digestion for biogas from lignocellulosic biomass? Bioresour. Technol. 2018, 249, 117–124. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Charnnok, B.; Sawangkeaw, R.; Chaiprapat, S. Integrated process for the production of fermentable sugar and methane from rubber wood. Bioresour. Technol. 2020, 302, 122785. [Google Scholar] [CrossRef]
- Pengilly, C.; García-Aparicio, M.P.; Diedericks, D.; Görgens, J.F. Optimization of enzymatic hydrolysis of steam pretreated triticale straw. BioEnergy Res. 2016, 9, 851–863. [Google Scholar] [CrossRef]
- Khan, M.U.; Ahring, B.K. Lignin degradation under anaerobic digestion: Influence of lignin modifications—A review. Biomass Bioenergy. 2019, 128, 105325. [Google Scholar] [CrossRef]
- Liew, L.N.; Shi, J.; Li, Y. Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass Bioenergy 2012, 46, 125–132. [Google Scholar] [CrossRef]
- Zverlov, V.V.; Schwarz, W.H. Bacterial cellulose hydrolysis in anaerobic environmental subsystems-Clostridium thermocellum and Clostridium stercorarium, thermophilic plant-fiber degraders. Ann. N. Y. Acad. Sci. 2008, 1125, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wen, Z. Enhancing enzymatic digestibility of switchgrass by microwave-assisted alkali pretreatment. Biochem. Eng. J. 2008, 38, 369–378. [Google Scholar] [CrossRef]
- Binod, P.; Satyanagalakshmi, K.; Sindhu, R.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renew. Energy 2012, 37, 109–116. [Google Scholar] [CrossRef]
- Sabiha-Hanim, S.; Noor, M.A.M.; Rosma, A. Fractionation of oil palm frond hemicelluloses by water or alkaline impregnation and steam explosion. Carbohydr. Polym. 2015, 115, 533–539. [Google Scholar] [CrossRef]
- Ni Sun, S.; Chen, X.; Tao, Y.H.; Cao, X.F.; Li, M.F.; Wen, J.L.; Nie, S.X.; Sun, R.C. Pretreatment of Eucalyptus urophylla in γ-valerolactone/dilute acid system for removal of non-cellulosic components and acceleration of enzymatic hydrolysis. Ind. Crops Prod. 2019, 132, 21–28. [Google Scholar] [CrossRef]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Inhibition of anaerobic digestion by dissolved lignin derived from alkaline pre-treatment of an aquatic macrophyte. Chem. Eng. J. 2017, 311, 55–62. [Google Scholar] [CrossRef]
- Li, L.; Kong, X.; Yang, F.; Li, D.; Yuan, Z.; Sun, Y.M. Biogas production potential and kinetics of microwave and conventional thermal pretreatment of grass. Appl. Biochem. Biotechnol. 2011, 166, 1183–1191. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Lira, I.O.H.-D.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into pretreatment methods of lignocellulosic biomass to increase biogas yield: current state, challenges, and opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef] [Green Version]
- Kainthola, J.; Shariq, M.; Kalamdhad, A.; Goud, V.V. Enhanced methane potential of rice straw with microwave assisted pretreatment and its kinetic analysis. J. Environ. Manag. 2019, 232, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef] [Green Version]




| Component | Unit | Napier Grass Silage |
|---|---|---|
| Ultimate analysis | ||
| Carbon (C) | % dry wt. | 45.31 ± 0.11 |
| Hydrogen (H) | % dry wt. | 5.82 ± 0.11 |
| Oxygen (O) | % dry wt. | 40.27 ± 0.73 |
| Nitrogen (N) | % dry wt. | 0.58 ± 0.02 |
| Sulphur (S) | % dry wt. | n.d. |
| Proximate analysis | ||
| Moisture | % fresh wt. | 76.48 ± 2.47 |
| Total Solids | % fresh wt. | 23.52 ± 2.47 |
| Volatile Solids | % fresh wt. | 21.31 ± 2.27 |
| Fiber composition | ||
| Cellulose | % dry wt. | 43.78 ± 0.37 |
| Hemicellulose | % dry wt. | 35.29 ± 1.11 |
| Lignin | % dry wt. | 4.36 ± 0.82 |
| Ash | % dry wt. | 1.03 ± 0.03 |
| Source | Predicted Methane Yield (%) | |
|---|---|---|
| Coefficient Estimate | Probability | |
| b0 | 82.1401 | <0.0001 |
| Temp | 4.0080 | <0.0001 |
| DT | −0.0548 | 0.0039 |
| Temp*DT | 0.0092 | 0.2385 |
| Temp2 | −0.0258 | <0.0001 |
| DT2 | −0.0009 | 0.9116 |
| F-significant | <0.0001 | |
| R2 | 0.8364 | |
| R2 adjusted | 0.8131 | |
| Coefficient of variance (CV) | 4.28 | |
| Method | Energy Intensity | Predicted Methane Yield | BI a | Energy Yield from CH4 in Biogas Produced | Increased Energy Gain ① | Energy Input b ② | ER c = Output/Input ①/② | |
|---|---|---|---|---|---|---|---|---|
| (kJ/kgsubstrate) | (mLCH4/gVS) | (%) | (MJ/kgVS) | (MJ/kgsubstrate) | (MJ/kgsubstrate) | (MJ/kgsubstrate) | ||
| Untreated | Untreated | 189.8 | 39.7 | 6.79 | 6.16 | - | - | - |
| CHTP | 3006 3443 4552 | 227.8 235.9 265.2 | 47.5 49.4 55.5 | 8.22 8.65 9.49 | 7.45 7.84 8.52 | 1.29 1.68 2.36 | 3.0 3.4 4.6 | 0.43 0.49 0.52 |
| MWP | 3006 3443 | 265.2 250.8 | 55.5 52.5 | 9.49 8.98 | 8.52 8.14 | 2.36 1.98 | 3.0 3.4 | 0.79 0.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saritpongteeraka, K.; Kaewsung, J.; Charnnok, B.; Chaiprapat, S. Comparing Low-Temperature Hydrothermal Pretreatments through Convective Heating versus Microwave Heating for Napier Grass Digestion. Processes 2020, 8, 1221. https://doi.org/10.3390/pr8101221
Saritpongteeraka K, Kaewsung J, Charnnok B, Chaiprapat S. Comparing Low-Temperature Hydrothermal Pretreatments through Convective Heating versus Microwave Heating for Napier Grass Digestion. Processes. 2020; 8(10):1221. https://doi.org/10.3390/pr8101221
Chicago/Turabian StyleSaritpongteeraka, Kanyarat, Jutawan Kaewsung, Boonya Charnnok, and Sumate Chaiprapat. 2020. "Comparing Low-Temperature Hydrothermal Pretreatments through Convective Heating versus Microwave Heating for Napier Grass Digestion" Processes 8, no. 10: 1221. https://doi.org/10.3390/pr8101221