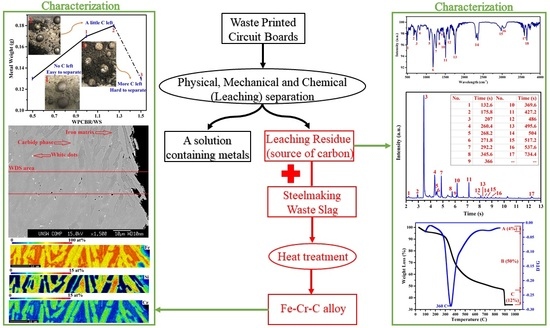
Thermal Isolation of a Clean Alloy from Waste Slag and Polymeric Residue of Electronic Waste
Abstract
:1. Introduction
2. Experimental
2.1. Feed Materials Preparation and Experiments
2.2. Characterization
- (a)
- X-ray diffraction analysis (XRD, PANalytical X’Pert Pro, Malvern, UK) with Cu Kα wavelength and 2θ between 10–100° was applied for phase identification of waste materials and the resulting alloy.
- (b)
- To increase the precision of phase identification, X-ray fluorescence analysis (XRF, PANalytical PW2400 Sequential Wavelength Dispersive, Malvern, UK) was utilized to quantify the approximate percentage of oxide phases.
- (c)
- Scanning electron microscopy (SEM, Hitachi S3400, Tokyo, Japan) equipped with energy dispersive spectroscopy (EDS, Bruker) was used for the morphological observation and distribution analysis of elements in the WPCBR.
- (d)
- LECO analysis was employed to measure the sulfur (S), nitrogen (N) (LECO TruSpec Analyser, Michigan, USA), and carbon contents (LECO CS 444 Analyser, Michigan, USA) of the WPCBR.
- (e)
- The structural and elemental concentrations of the recycled metal were measured by electron probe microanalysis (EPMA) or wavelength dispersive spectroscopy (WDS, JEOL JXA-8500F) for accurate quantitative mapping and point analysis. The sample was mirror polished and all measured elements were calibrated using standard samples.
- (f)
- Moreover, the composition of the recycled metal was analyzed using laser-induced breakdown spectroscopy (LIBS, Z-200 under Ar atmosphere), which is known as a very reliable method even for carbon content analysis. The surface of the sample was mirror polished and the device was calibrated before analysis.
- (g)
- The thermal behavior and proximate analysis of the WPCBR were carried out using thermogravimetric analysis (TGA, PerkinElmer STA 8000 and TGA 8000, Massachusetts, USA) and differential thermogravimetric (DTG) in an alumina crucible under a controlled atmosphere (N2 or O2) at a heating rate of 20 °C/min.
- (h)
- The exhaust of the TGA was connected to Fourier transform infrared spectroscopy (FT-IR, PerkinElmer, Frontier, Massachusetts, USA) with continuous wavelength analysis between 4000 and 500 cm−1 to identify the functional groups of any evaporated organic species and to measure the volume of the evolved gas.
- (i)
- The exhaust of the FT-IR was joined to a gas chromatograph-mass spectrometer (GC/MS, PerkinElmer, Clatus 500 and Clarus SQ 8 s, Massachusetts, USA) for more accurate recognition of organic species based on separation time. TurboMass V 6.1 equipped with the National Institute of Standards and Technology (NIST) library was used for the identification of the compounds.
3. Result and Discussion
3.1. Characterization of Waste Materials
3.2. Reduction and Recycling Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yildirim, I.Z.; Mrezzi, M. Experimental evaluation of EAF ladle steel slag as a geo-fill material: Mineralogical, physical & mechanical properties. Constr. Build. Mater. 2017, 154, 23–33. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Rajarao, R.; Golmohammadzadeh, R.; Sahajwalla, V. Direct transformation of waste printed circuit boards to nano-structured powders through mechanical alloying. Mater. Des. 2018, 141, 26–36. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.; Guan, D. Take responsibility for electronic-waste disposal. Nature 2016, 536, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, I.Z.; Prezzi, M. Chemical, mineralogical, and morphological properties of steel slag. Adv. Civ. Eng. 2011, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nekouei, R.K.; Pahlevani, F.; Rajarao, R.; Golmohammadzadeh, R.; Sahajwalla, V. Two-step pre-processing enrichment of waste printed circuit boards: Mechanical milling and physical separation. J. Clean. Prod. 2018, 184, 1113–1124. [Google Scholar] [CrossRef]
- Kumar, A.; Holuszko, M.; Espinosa, D.C.R. E-waste: An overview on generation, collection, legislation and recycling practices. Resour. Conserv. Recycl. 2017, 122, 32–42. [Google Scholar] [CrossRef]
- Song, Q.; Li, J. Environmental effects of heavy metals derived from the e-waste recycling activities in China: A systematic review. Waste Manag. 2014, 34, 2587–2594. [Google Scholar] [CrossRef]
- Singh, N.; Li, J.; Zeng, X. Global responses for recycling waste CRTs in e-waste. Waste Manag. 2016, 57, 187–197. [Google Scholar] [CrossRef]
- Sahajwalla, V.; Gaikwad, V. The present and future of e-waste plastics recycling. Curr. Opin. Green Sustain. Chem. 2018, 13, 102–107. [Google Scholar] [CrossRef]
- Senophiyah-Mary, J.; Loganath, R.; Shameer, P.M. Deterioration of cross linked polymers of thermoset plastics of e-waste as a side part of bioleaching process. J. Environ. Chem. Eng. 2018, 6, 3185–3191. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic Pollutants from Plastic Waste—A Review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Maroufi, S.; Mayyas, M.; Nekouei, R.K.; Assefi, M.; Sahajwalla, V. Thermal Nanowiring of E-Waste: A Sustainable Route for Synthesizing Green Si3N4Nanowires. ACS Sustain. Chem. Eng. 2018, 6, 3765–3772. [Google Scholar] [CrossRef]
- Skaf, M.; Manso, J.M.; Aragón, Á.; Fuente-Alonso, J.A.; Ortega-López, V. EAF slag in asphalt mixes: A brief review of its possible re-use. Resour. Conserv. Recycl. 2017, 120, 176–185. [Google Scholar] [CrossRef]
- Ortega-López, V.; Fuente-Alonso, J.A.; Santamaría, A.; San-José, J.T.; Aragón, Á. Durability studies on fiber-reinforced EAF slag concrete for pavements. Constr. Build. Mater. 2018, 163, 471–481. [Google Scholar] [CrossRef]
- Zuo, M.; Renman, G.; Gustafsson, J.P.; Klysubun, W. Phosphorus removal by slag depends on its mineralogical composition: A comparative study of AOD and EAF slags. J. Water Process Eng. 2018, 25, 105–112. [Google Scholar] [CrossRef]
- Xu, R.; Dai, B.; Wang, W.; Schenk, J.; Xue, Z. Effect of iron ore type on the thermal behaviour and kinetics of coal-iron ore briquettes during coking. Fuel Process. Technol. 2018, 173, 11–20. [Google Scholar] [CrossRef]
- Liou, T.-H.; Jheng, J.-Y. Synthesis of High-Quality Ordered Mesoporous Carbons Using a Sustainable Way from Recycling of E-waste as a Silica Template Source. ACS Sustain. Chem. Eng. 2018, 6, 6507–6517. [Google Scholar] [CrossRef]
- Dong, H.; Geng, Y.; Yu, X.; Li, J. Uncovering energy saving and carbon reduction potential from recycling wastes: A case of Shanghai in China. J. Clean. Prod. 2018, 205, 27–35. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Golmohammadzadeh, R.; Assefi, M.; Rajarao, R.; Chen, Y.-H.; Sahajwalla, V. Recovery of heavy metals from waste printed circuit boards: Statistical optimization of leaching and residue characterization. Environ. Sci. Pollut. Res. 2019, 1–13. [Google Scholar] [CrossRef]
- Maroufi, S.; Nekouei, R.K.; Hassan, K.; Sahajwalla, V. Thermal Transformation of Mixed E-Waste Materials into Clean SiMn/FeMn Alloys. ACS Sustain. Chem. Eng. 2018, 6, 13296–13301. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Li, X. Steel-making slag for mineral sequestration of carbon dioxide by accelerated carbonation. Meas. J. Int. Meas. Confed. 2017, 97, 15–22. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Hao, J.; He, Y.; Zhang, T.; Yang, X. Morphology, mineralogy and separation characteristics of nonmetallic fractions from waste printed circuit boards. J. Clean. Prod. 2018, 170, 1501–1507. [Google Scholar] [CrossRef]
- Shokri, A.; Pahlevani, F.; Levick, K.; Cole, I.; Sahajwalla, V. Synthesis of copper-tin nanoparticles from old computer printed circuit boards. J. Clean. Prod. 2017, 142, 2586–2592. [Google Scholar] [CrossRef]
- Maroufi, S.; Nekouei, S.K.; Assefi, M.; Sahajwalla, V. Waste-cleaning waste: Synthesis of ZnO porous nano-sheets from batteries for dye degradation. Environ. Sci. Pollut. Res. 2018, 25, 28594–28600. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Yang, F.; Lin, W.; Li, S.; Sun, S. Improvement of pyrolysis oil obtained from co-pyrolysis of WPCBs and compound additive during two stage pyrolysis. J. Anal. Appl. Pyrolysis 2018, 135, 415–421. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Preserova, J.; Ranc, V.; Milde, D.; Kubistova, V.; Stavek, J. Study of phenolic profile and antioxidant activity in selected Moravian wines during winemaking process by FT-IR spectroscopy. J. Food Sci. Technol. 2015, 52, 6405–6414. [Google Scholar] [CrossRef] [Green Version]
- Biber, M.V.; Stumm, W. An In-Situ ATR-FTIR Study: The Surface Coordination of Salicylic Acid on Aluminum and Iron(III) Oxides. Environ. Sci. Technol. 1994, 28, 763–768. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Zhihua, L.; Jiyong, S.; Zhai, X.; Wang, S.; Mariod, A.A. Rapid prediction of phenolic compounds and antioxidant activity of Sudanese honey using Raman and Fourier transform infrared (FT-IR) spectroscopy. Food Chem. 2017, 226, 202–211. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Maroufi, S.; Mayyas, M.; Mansuri, I.; O’Kane, P.; Skidmore, C.; Jin, Z.; Fontana, A.; Sahajwalla, V. Study of Reaction Between Slag and Carbonaceous Materials. Metall. Mater. Trans. B 2017, 48, 2316–2323. [Google Scholar] [CrossRef]
- Help of HSC V.9 Software Website. Available online: https://www.outotec.com/products/digital-solutions/hsc-chemistry/ (accessed on 1 June 2019).
- Fruehan, R.J. Rate of Reduction of Cr2O3 by Carbon and Carbon Dissolved in Liquid Iron Alloys. Metall. Trans. B 1977, 8, 429–433. [Google Scholar] [CrossRef]
- Şeşen, F.E. Practical reduction of manganese oxide. J. Chem. Tech. App. 2017, 1, 1–2. [Google Scholar]
- Farzana, R.; Rajarao, R.; Sahajwalla, V. Reaction Mechanism of Ferrosilicon Synthesis Using Waste Plastic as a Reductant. ISIJ Int. 2017, 57, 1780–1787. [Google Scholar] [CrossRef] [Green Version]
- Maroufi, S.; Nekouei, R.K.; Hossain, R.; Assefi, M.; Sahajwalla, V. Recovery of Rare Earth (i.e., La, Ce, Nd, and Pr) Oxides from End-of-Life Ni-MH Battery via Thermal Isolation. ACS Sustain. Chem. Eng. 2018, 6, 11811–11818. [Google Scholar] [CrossRef]
- Schrama, F.N.H.; Beunder, E.M.; Van den Berg, B.; Yang, Y.; Boom, R. Sulphur removal in ironmaking and oxygen steelmaking. Ironmak. Steelmak. 2017, 44, 333–343. [Google Scholar] [CrossRef]
- Penn, C.; Chagas, I.; Klimeski, A.; Lyngsie, G. A review of phosphorus removal structures: How to assess and compare their performance. Water (Switzerland) 2017, 9, 583. [Google Scholar] [CrossRef]









| Phase (wt%) Waste Material | Fe2O3 | SiO2 | MnO | CaO | Al2O3 | MgO | Na2O | P2O5 | Cr2O3 |
|---|---|---|---|---|---|---|---|---|---|
| Ash content of WPCBR | 0.9 | 61.3 | 0.1 | 15.5 | 11.8 | 0.7 | 0.7 | 0.1 | 0.1 |
| WS | 38.6 | 12.0 | 7.2 | 30.0 | 4.3 | 7.0 | 0.3 | 0.4 | 2.0 |
| Phase or Element | N 1 | S 1 | Total Carbon 1 | Total Volatile 2 | Fixed Carbon 2 | Ash 2 |
|---|---|---|---|---|---|---|
| Weight Percentage (wt%) | 2.2 | 0.08–0.1 | 29.3 | 54 | 12 | 34 |
| No. | Functional Group | Absorption Location (cm−1) | Type of Vibration |
|---|---|---|---|
| 1 | C‒Br | 502 | Stretching |
| 2 | C=C alkene | 664 | Bending |
| 3 | C‒H | 748 | Bending |
| 4 | C‒H | 829 | Bending |
| 5 | C‒O‒C | 1086 | Stretching |
| 6 | C‒O‒C | 1177 | Stretching |
| 7 | C‒O phenolic | 1258 | Stretching |
| 8 | C‒O phenolic | 1339 | Bending |
| 9 | C‒H aliphatic | 1417 | Bending |
| 10 | C=C | 1487 | Stretching |
| 11 | C=C | 1504 | Stretching |
| 12 | C=C benzene | 1603 | Stretching |
| 13 | C=O carboxylic acid | 1756 | Stretching |
| 14 | O=C=O carbon dioxide | 2356, 2310 | Stretching |
| 15 | O‒H phenol (H-bond) | 2974 | Stretching |
| 16 | O‒H carboxylic acid (H-bond) | 3032 | Stretching |
| 17 | O‒H | 3588 | Stretching |
| 18 | O‒H phenol | 3649 | Stretching |
| Element | Fe | Si | Cr | Mn | C |
|---|---|---|---|---|---|
| Weight Percent (%) | 88.9 ± 0.8 | 1.7 ± 0.1 | 3.9 + 0.1 | 1.3 ± 0.1 | 3.8 ± 0.4 |
| Element Phase | C | Mn | Si | Cr | Br | P | Fe |
|---|---|---|---|---|---|---|---|
| Atomic Percent (at%) | |||||||
| Iron Matrix | 1.16 ± 0.04 | 1.79 ± 0.06 | 11.82 ± 0.23 | 0.92 ± 0.04 | <0.002 | 0.84 ± 0.06 | 83.46 ± 0.19 |
| Carbide Phase | 26.62 ± 0.17 | 3.35 ± 0.23 | 0.05 ± 0.02 | 6.34 ± 0.71 | <0.002 | 0.00 ± 0.00 | 63.65 ± 0.60 |
| White Dots | 3.65 ± 0.45 | 2.15 ± 0.50 | 9.32 ± 0.19 | 1.16 ± 0.35 | <0.008 | 1.99 ± 0.67 | 81.73 ± 2.15 |
| Weight Percent (wt%) | |||||||
| Iron Matrix | 0.27 ± 0.01 | 1.90 ± 0.06 | 6.41 ± 0.14 | 0.92 ± 0.05 | <0.003 | 0.50 ± 0.03 | 89.94 ± 0.27 |
| Carbide Phase | 7.29 ± 0.08 | 4.20 ± 0.29 | 0.03 ± 0.01 | 7.51 ± 0.84 | <0.003 | 0.00 ± 0.00 | 81.08 ± 0.76 |
| White Dots | 0.84 ± 0.10 | 2.26 ± 0.51 | 5.02 ± 0.06 | 1.15 ± 0.34 | <0.013 | 1.18 ± 0.39 | 87.53 ± 3.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khayyam Nekouei, R.; Maroufi, S.; Assefi, M.; Pahlevani, F.; Sahajwalla, V. Thermal Isolation of a Clean Alloy from Waste Slag and Polymeric Residue of Electronic Waste. Processes 2020, 8, 53. https://doi.org/10.3390/pr8010053
Khayyam Nekouei R, Maroufi S, Assefi M, Pahlevani F, Sahajwalla V. Thermal Isolation of a Clean Alloy from Waste Slag and Polymeric Residue of Electronic Waste. Processes. 2020; 8(1):53. https://doi.org/10.3390/pr8010053
Chicago/Turabian StyleKhayyam Nekouei, Rasoul, Samane Maroufi, Mohammad Assefi, Farshid Pahlevani, and Veena Sahajwalla. 2020. "Thermal Isolation of a Clean Alloy from Waste Slag and Polymeric Residue of Electronic Waste" Processes 8, no. 1: 53. https://doi.org/10.3390/pr8010053