Response Surface Method in the Optimization of a Rotary Pan-Equipped process for Increased Efficiency of Slow-Release Coated Urea
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Related Analysis
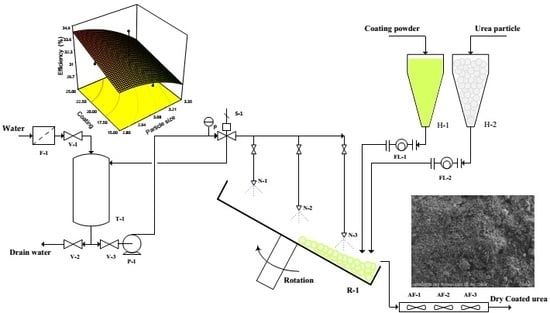
2.2. Coating Process
2.3. Analysis of Urea Dissolution
2.4. Experimental Design
2.5. Characterization Method
3. Results and Discussion
3.1. Normality Tests
3.2. Analysis of Variance (ANOVA)
3.3. Interaction Effects between the Process Variables
3.4. Response Surface and Process Optimisation
3.5. Structural Properties of the Coated Urea
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Simmons, C.L.; Cole, R.D. Sulfur coating of urea containing gelling clays. US Patent H1085, 8 April 1992. [Google Scholar]
- Blouin, G.M. Production of high-strength, storage-stable particulate urea. US Patent 4587358, 6 May 1986. [Google Scholar]
- Gullett, L.L.; Simmons, C.L. Sulfur-coated urea. US Patent 4676821, 30 June 1987. [Google Scholar]
- Savant, N.K.; Stangel, P.J. Deep placement of urea super granules in transplanted rice: Principles and practices. Fertil. Res. 1990, 25, 1–83. [Google Scholar] [CrossRef]
- Roy, B. Coated and modified urea materials for increasing nitrogen use efficiency of lowland rice in heavy clay soils. Fertil. Res. 1988, 15, 101–109. [Google Scholar] [CrossRef]
- Malhi, S.S.; Oliver, E.; Mayerle, G.; Kruger, G.; Gill, K.S. Improving effectiveness of seedrow-placed urea with urease inhibitor and polymer coating for durum wheat and canola. Comm. Soil Sci. Plant. Anal. 2003, 34, 1709–1727. [Google Scholar] [CrossRef]
- Junejo, N.; Khanif, M.Y.; Hanfi, M.M.; Wan, Z.W.Y.; Dharejo, K.A. Maize response to biodegradable polymer and urease inhibitor coated urea. Int. J. Agric. Biol. 2010, 12, 773–776. [Google Scholar]
- Salman, O.A. Polymer coating on urea prills to reduce dissolution rate. J. Agric. Food Chem. 1988, 36, 616–621. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Li, Y.; Zhao, B.; Zhang, L.; Yang, X.; Chang, J. Research on permeability coefficient of a polyethylene controlledrelease film coating for urea and relevant nutrient release pathways. Polym. Test. 2017, 59, 90–98. [Google Scholar] [CrossRef]
- Cahill, S.; Osmond, D.; Israel, D. Nitrogen release from coated urea fertilizers in different soils. Commun. Soil Sci. Plant Anal. 2010, 41, 1245–1256. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Gasparatos, D.; Zechmann, B.; Bouranis, L.D.; Chorianopoulou, S.N. The Effect of Granular Commercial Fertilizers Containing Elemental Sulfur on Wheat Yield under Mediterranean Conditions. Plants 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Blouin, G.M.; Rindt, D.W. Sulfur-coated fertilizer pellet having controlled dissolution rate and inhibited against microbial decomposition. US Patent 3342577, 19 September 1967. [Google Scholar]
- Rindt, D.W.; Blouin, G.M.; Getsinger, J.G. Sulfur coating on nitrogen fertilizer to reduce dissolution rate. J. Agric. Food Chem. 1968, 16, 773–778. [Google Scholar] [CrossRef]
- Blouin, G.M.; Rindt, D.W.; Moore, O.E. Sulfur-coated fertilizers for controlled release. Pilot-plant production. J. Agric. Food Chem. 1971, 19, 801–808. [Google Scholar] [CrossRef]
- Shirley, A.R.; Meline, R.S. Production of slow release nitrogen fertilizers by improved method of coating urea with sulfur. US Patent 3903333A, 2 September 1975. [Google Scholar]
- Meisen, A.; Mathur, K.B. Production of sulphur-coated urea by the spouted bed process. In Proceedings of the British Sulfur Corporation-Part I, London, UK, 3–6 December 1978; p. XTV-2. [Google Scholar]
- Salman, O.A.; Hovakeemian, G.; Khraishi, N. Polyethylene-coated urea. 2. Urea release as affected by coating material, soil type and temperature. Ind. Eng. Chem. Res. 1989, 28, 633–638. [Google Scholar] [CrossRef]
- Gullett, L.L.; Simmons, C.L.; Lee, R.G. Sulfur coating of urea treated with attapulgite clay. Fertil. Res. 1991, 28, 123–128. [Google Scholar] [CrossRef]
- Goertz, H.M.; Timmons, R.J.; McVey, G.R. Sulfur coated fertilizers and process for the preparation thereof. US Patent 5219465, 15 June 1993. [Google Scholar]
- Choi, M.; Meisen, A. Sulfur coating of urea in shallow spouted beds. Chem. Eng. Sci. 1997, 52, 1073–1086. [Google Scholar] [CrossRef]
- Ayub, G.S.E.; Rocha, S.C.S.; Perrucci, A.L.I. Analysis of the Surface Quality of Sulphur- Coated Urea Particles in a Two-Dimensional Spouted Bed. Braz. J. Chem. Eng. 2001, 18, 13–22. [Google Scholar] [CrossRef]
- Donida, M.; Rocha, S.C.S. Coating of urea with an aqueous polymeric suspension in a two-dimensional spouted bed. Drying Technol. 2002, 20, 37–41. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.-J.; Qin, L.; Jin, Y. Urea particle coating for controlled release by using DCPD modified sulfur. Powder Technol. 2008, 183, 88–93. [Google Scholar] [CrossRef]
- da Rosa, G.S.; dos Santos Rocha, S.C. Effect of process conditions on particle growth for spouted bed coating of urea. Chem. Eng. Process. 2010, 49, 836–842. [Google Scholar] [CrossRef]
- Mulder, W.J.; Gosselink, R.J.; Vingerhoeds, M.H.; Harmsen, P.F.H.; Eastham, D. Lignin based controlled release coating. Ind. Crops Prod. 2011, 34, 915–920. [Google Scholar] [CrossRef]
- Lan, R.; Liu, Y.; Wang, G.; Wang, T.K.; Kan, C.; Jin, Y. Experimental modeling of polymer latex spray coating for producing controlled-release urea. Particuology 2011, 9, 510–516. [Google Scholar] [CrossRef]
- Qin, S.; Wu, Z.; Rasool, A.; Li, C. Synthesis and characterization of slow-release nitrogen fertilizer with water absorbency: Based on poly(acrylic acid-acrylic amide)/Na-bentonite. J. Appl. Polym. Sci. 2012, 126, 1687–1697. [Google Scholar] [CrossRef]
- Pursell, T.; Shirley, A.R.; Cochran, K.D.; Miller, J.M.; Holt, T.G.; Peeden, G.S. Controlled Release Fertilizer with Biopolymer Coating and Process for Making Same. US Patent 9266787 B2, 23 February 2016. [Google Scholar]
- Guo, Y.; Zhang, M.; Liu, Z.; Tian, X.; Zhang, S.; Zhao, C.; Lu, H. Modeling and Optimizing the Synthesis of Urea-formaldehyde Fertilizers and Analyses of Factors Affecting these Processes. Sci. Rep. 2018, 8, 4504. [Google Scholar] [CrossRef] [PubMed]
- Bah, A.R.; Rahman, Z.A. Evaluating urea fertilizer formulations for oil palm seedlings using the 15N isotope dilution technique. J. Oil Palm Res. 2004, 16, 72–77. [Google Scholar]
- Weisany, W.; Raei, Y.; Haji Allahverdipoor, K. Role of Some of Mineral Nutrients in Biological Nitrogen Fixation. Bull. Env. Pharmacol. Life Sci. 2013, 2, 77–84. [Google Scholar]
- Eghbali Babadi, F.; Yunus, R.; Abdul Rashid, S.; Mohd Salleh, M.A.; Ali, S. New coating formulation for the slow release of urea using a mixture of gypsum and dolomitic limestone. Particuology 2015, 23, 62–67. [Google Scholar] [CrossRef]
- George, D.; Mallery, M. SPSS for Windows Step by Step: A Simple Guide and Reference, 10th ed.; 17.0 update; Allyn & Bacon: Boston, MA, USA, 2009; pp. 1–400. [Google Scholar]
- Traub, R.E.; Rowley, G.L. An NCME instructional module on understanding reliability. Educ. Meas. 1991, 10, 37–45. [Google Scholar] [CrossRef]
- Nathans, L.L.; Oswald, F.L.; Nimon, K. Interpreting multiple linear regression: A guidebook of variable importance. Pract. Assess. Res. Eval. 2012, 17, 1–19. [Google Scholar]
- Sahni, E.; Chaudhuri, B. Experiments and numerical modeling to estimate the coating variability in a pan coater. Int. J. Pharm. 2011, 418, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Obara, S.; McGinity, J.W. Influence of processing variables on the properties of free films prepared from aqueous polymeric dispersions by a spray technique. Int. J. Pharm. 1995, 126, 1–10. [Google Scholar] [CrossRef]
- Marple, B.R.; Hyland, M.M.; Lau, Y.C.; Li, C.J.; Lima, R.S.; Montavon, G. Thermal Spray 2007: Global Coating Solutions. In Proceedings of the 2007 International Thermal Spray Conference, Beijing, China, 14–16 May 2007. [Google Scholar]
- Tzika, M.; Alexandridou, S.; Kiparissides, C. Evaluation of the morphological and release characteristics of coated fertilizer granules produced in a Wurster fluidized bed. Powder Technol. 2003, 132, 16–24. [Google Scholar] [CrossRef]
- Wong, P.M.; Chan, L.W.; Heng, P.W.S. Investigation on side-spray fluidized bed granulation with swirling airflow. AAPS PharmSciTech. 2013, 14, 211–221. [Google Scholar] [CrossRef] [PubMed]













| Variables | Units | Coded Level of Variables | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Particle size (X1) | mm | 2.80 | 3.10 | 3.35 |
| Coating percentage (X2) | % | 15 | 20 | 25 |
| Pan speed (X3) | rpm | 8 | 12 | 16 |
| Spray flow rate (X4) | g/min | 50 | 75 | 100 |
| Spray water (X5) | % | 1.4 | 1.5 | 1.6 |
| Descriptive Statistics | Particle Size (mm) | Coating (%) | Pan Speed (rpm) | Spray Flow Rate (g/min) | Spray Water (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −1 | 0 | +1 | −1 | 0 | +1 | −1 | 0 | +1 | −1 | 0 | +1 | −1 | 0 | +1 | |
| Skewness | −0.447 | −0.740 | −0.059 | −0.638 | −0.779 | 0.429 | 0.150 | −1.108 | −0.226 | −0.601 | −0.780 | 0.194 | −0.365 | −0.780 | −0.128 |
| Kurtosis | 0.177 | 3.902 | −0.819 | 0.556 | 2.571 | −1.278 | −1.123 | 4.020 | −0.269 | −0.015 | 2.584 | −0.079 | −0.238 | 2.584 | −0.091 |
| Run | X1 | X2 | X3 | X4 | X5 | Efficiency % | |
|---|---|---|---|---|---|---|---|
| Actual | Predicted | ||||||
| 1 | 2.8 | 15 | 8 | 50 | 1.4 | 27.271 | 27.014 |
| 2 | 3.35 | 15 | 8 | 50 | 1.4 | 26.155 | 26.628 |
| 3 | 2.8 | 25 | 8 | 50 | 1.4 | 28.268 | 28.998 |
| 4 | 3.35 | 25 | 8 | 50 | 1.4 | 28.101 | 28.351 |
| 5 | 2.8 | 15 | 16 | 50 | 1.4 | 27.479 | 27.292 |
| 6 | 3.35 | 15 | 16 | 50 | 1.4 | 22.536 | 22.575 |
| 7 | 2.8 | 25 | 16 | 50 | 1.4 | 32.036 | 31.930 |
| 8 | 3.35 | 25 | 16 | 50 | 1.4 | 27.235 | 26.951 |
| 9 | 2.8 | 15 | 8 | 100 | 1.4 | 26.573 | 26.678 |
| 10 | 3.35 | 15 | 8 | 100 | 1.4 | 26.953 | 26.781 |
| 11 | 2.8 | 25 | 8 | 100 | 1.4 | 28.973 | 28.654 |
| 12 | 3.35 | 25 | 8 | 100 | 1.4 | 27.073 | 27.081 |
| 13 | 2.8 | 15 | 16 | 100 | 1.4 | 27.324 | 26.956 |
| 14 | 3.35 | 15 | 16 | 100 | 1.4 | 22.262 | 22.727 |
| 15 | 2.8 | 25 | 16 | 100 | 1.4 | 32.478 | 31.585 |
| 16 | 3.35 | 25 | 16 | 100 | 1.4 | 25.136 | 25.681 |
| 17 | 2.8 | 15 | 8 | 50 | 1.6 | 28.327 | 28.408 |
| 18 | 3.35 | 15 | 8 | 50 | 1.6 | 28.187 | 28.023 |
| 19 | 2.8 | 25 | 8 | 50 | 1.6 | 29.136 | 28.288 |
| 20 | 3.35 | 25 | 8 | 50 | 1.6 | 27.523 | 27.642 |
| 21 | 2.8 | 15 | 16 | 50 | 1.6 | 28.477 | 28.687 |
| 22 | 3.35 | 15 | 16 | 50 | 1.6 | 24.510 | 23.970 |
| 23 | 2.8 | 25 | 16 | 50 | 1.6 | 31.233 | 31.220 |
| 24 | 3.35 | 25 | 16 | 50 | 1.6 | 26.600 | 26.241 |
| 25 | 2.8 | 15 | 8 | 100 | 1.6 | 28.102 | 28.073 |
| 26 | 3.35 | 15 | 8 | 100 | 1.6 | 28.651 | 28.175 |
| 27 | 2.8 | 25 | 8 | 100 | 1.6 | 27.893 | 27.944 |
| 28 | 3.35 | 25 | 8 | 100 | 1.6 | 26.969 | 26.371 |
| 29 | 2.8 | 15 | 16 | 100 | 1.6 | 27.988 | 28.351 |
| 30 | 3.35 | 15 | 16 | 100 | 1.6 | 23.903 | 24.122 |
| 31 | 2.8 | 25 | 16 | 100 | 1.6 | 29.725 | 30.875 |
| 32 | 3.35 | 25 | 16 | 100 | 1.6 | 24.974 | 24.971 |
| 33 | 2.8 | 20 | 12 | 75 | 1.5 | 32.843 | 33.175 |
| 34 | 3.35 | 20 | 12 | 75 | 1.5 | 29.909 | 30.384 |
| 35 | 3.08 | 15 | 12 | 75 | 1.5 | 31.163 | 31.503 |
| 36 | 3.08 | 25 | 12 | 75 | 1.5 | 32.601 | 33.273 |
| 37 | 3.08 | 20 | 8 | 75 | 1.5 | 31.517 | 32.669 |
| 38 | 3.08 | 20 | 16 | 75 | 1.5 | 32.238 | 32.108 |
| 39 | 3.08 | 20 | 12 | 50 | 1.5 | 29.036 | 29.891 |
| 40 | 3.08 | 20 | 12 | 100 | 1.5 | 29.489 | 29.441 |
| 41 | 3.08 | 20 | 12 | 75 | 1.4 | 30.603 | 30.573 |
| 42 | 3.08 | 20 | 12 | 75 | 1.6 | 30.080 | 30.916 |
| 43 | 3.08 | 20 | 12 | 75 | 1.5 | 33.601 | 32.388 |
| 44 | 3.08 | 20 | 12 | 75 | 1.5 | 31.276 | 32.388 |
| 45 | 3.08 | 20 | 12 | 75 | 1.5 | 32.043 | 32.388 |
| 46 | 3.08 | 20 | 12 | 75 | 1.5 | 34.809 | 32.388 |
| 47 | 3.08 | 20 | 12 | 75 | 1.5 | 32.894 | 32.388 |
| 48 | 3.08 | 20 | 12 | 75 | 1.5 | 33.352 | 32.388 |
| Source | Standard Deviation | R-Squared | Adjusted R-Squared | Predicted | PRESS * | Remarks |
|---|---|---|---|---|---|---|
| R-Squared | ||||||
| Linear | 2.68 | 0.2462 | 0.1565 | 0.0686 | 371.66 | - |
| 2FI | 2.71 | 0.4117 | 0.1359 | 0.0854 | 433.09 | - |
| Quadratic | 0.81 | 0.9552 | 0.9220 | 0.8942 | 42.21 | Suggested |
| Cubic | 1.05 | 0.9671 | 0.8711 | 0.3186 | 125.14 | Aliased |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 379.56 | 15 | 25.30 | 41.58 | <0.0001 a |
| Residual | 19.47 | 32 | 0.61 | - | - |
| Lack of fit | 11.82 | 27 | 0.44 | 0.29 | 0.9855 b |
| Pure error | 7.66 | 5 | 1.53 | - | - |
| Corrected total | 399.03 | 47 | - | - | - |
| R-Squared | 0.9512 | Standard Deviation | 0.78 | ||
| Adjusted R2 | 0.9283 | Coefficient of variation % | 2.70 | ||
| Adequate Precision | 23.753 | PRESS | 35.22 | ||
| Source | Coefficient Estimate | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|---|
| Intercept | 32.39 | - | 1 | - | - | - |
| X1 | 1.40 | 66.23 | 1 | 66.23 | 108.84 | < 0.0001 |
| X2 | 0.89 | 26.63 | 1 | 26.63 | 43.77 | < 0.0001 |
| X3 | −0.28 | 2.68 | 1 | 2.68 | 4.40 | 0.0440 |
| X4 | −0.22 | 1.72 | 1 | 1.72 | 2.82 | 0.1027 |
| X5 | 0.17 | 1.00 | 1 | 1.00 | 1.64 | 0.2097 |
| X1 X2 | −0.24 | 1.88 | 1 | 1.88 | 3.08 | 0.0887 |
| X1 X3 | −1.08 | 37.53 | 1 | 37.53 | 61.67 | < 0.0001 |
| X1 X4 | −0.05 | 0.10 | 1 | 0.10 | 0.16 | 0.6938 |
| X2 X3 | 0.66 | 14.08 | 1 | 14.08 | 23.13 | < 0.0001 |
| X2 X4 | −0.18 | 1.02 | 1 | 1.02 | 1.68 | 0.2037 |
| X2 X5 | −0.53 | 8.86 | 1 | 8.86 | 14.56 | 0.0006 |
| X12 | −0.61 | 1.07 | 1 | 1.07 | 1.76 | 0.1943 |
| X42 | −2.72 | 21.40 | 1 | 21.40 | 35.17 | < 0.0001 |
| X52 | −1.64 | 7.80 | 1 | 7.80 | 12.82 | 0.0011 |
| X1 X2 X4 | −0.18 | 1.00 | 1 | 1.00 | 1.64 | 0.2094 |
| Optimal condition | Efficiency % | ||||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | Actual | Predicted | Relative Deviation |
| 2.80 | 25.00 | 16.00 | 73.00 | 1.50 | 34.56 | 35.79 | 3.44 |
| Sample | Total Surface Area (m2/g) | Total Pore Volume (cm³/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Urea | 3.5397 | 0.173146 | 57.4 |
| Non-optimum coated urea | 1.8238 | 0.162450 | 58.2 |
| Optimum coated urea | 1.7048 | 0.147391 | 53.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eghbali Babadi, F.; Yunus, R.; Abbasi, A.; Masoudi Soltani, S. Response Surface Method in the Optimization of a Rotary Pan-Equipped process for Increased Efficiency of Slow-Release Coated Urea. Processes 2019, 7, 125. https://doi.org/10.3390/pr7030125
Eghbali Babadi F, Yunus R, Abbasi A, Masoudi Soltani S. Response Surface Method in the Optimization of a Rotary Pan-Equipped process for Increased Efficiency of Slow-Release Coated Urea. Processes. 2019; 7(3):125. https://doi.org/10.3390/pr7030125
Chicago/Turabian StyleEghbali Babadi, Farahnaz, Robiah Yunus, Ali Abbasi, and Salman Masoudi Soltani. 2019. "Response Surface Method in the Optimization of a Rotary Pan-Equipped process for Increased Efficiency of Slow-Release Coated Urea" Processes 7, no. 3: 125. https://doi.org/10.3390/pr7030125