Flocculation of a High-Turbidity Kaolin Suspension Using Hydrophobic Modified Quaternary Ammonium Salt Polyacrylamide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PAMD
2.3. Flocculation Experiments
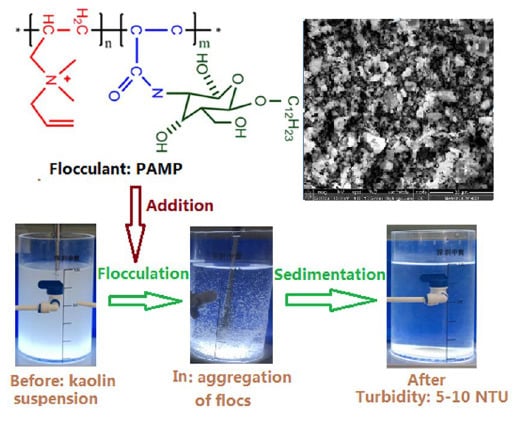
3. Results and Discussion
3.1. OptimalSynthesis Conditions
3.1.1. Effect of Solid Content
3.1.2. Effect of DMD Content
3.1.3. Effect of DPL Content
3.1.4. Effect of Illumination Time
3.1.5. Effect of pH
3.2. Flocculation Performance
3.2.1. Effect of DMD Content and Dosage
3.2.2. Effect of DPL Content and Dosage
3.2.3. Effect of Deposition Time and pH
3.2.4. Effect of Stirring Speed and Stirring Time
3.2.5. Comparison of Flocculation Performance between PAMD and PAC
3.3. Response Surface Method
3.4. Turbidity Removal Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Knaeps, E.; Ruddick, K.G.; Doxaran, D.; Dogliotti, A.I.; Nechad, B.; Raymaekers, D.; Sterckx, S. A SWIR based algorithm to retrieve total suspended matter in extremely turbid waters. Remote Sens. Environ. 2015, 168, 66–70. [Google Scholar] [CrossRef]
- Mores, R.; Treichel, H.; Zakrzevski, C.A.; Kunz, A.; Steffens, J.; Dallago, R.M. Remove of phosphorous and turbidity of swine wastewater using electro coagulation under continuous flow. Sep. Purif. Technol. 2016, 171, 112–115. [Google Scholar] [CrossRef]
- Zahrim, A.Y.; Tizaoui, C.; Hilal, N. Coagulation with polymers for nano filtration pre-treatment of highly concentrated dyes: A review. Desalination 2011, 266, 1–5. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Arena, F.; Chio, R.D.; Gumina, B.; Spadaro, L.; Trunfio, G. Recent advances on wet air oxidation catalysts for treatment of industrial wastewaters. Inorg. Chim. Acta 2015, 431, 101–109. [Google Scholar] [CrossRef]
- Guan, Q.Q.; Zhu, G.C.; Liao, Y.; Xu, J.; Sun, X.X.; Tian, F.; Xu, J.X.; Luo, M.H. Preparation, characterization, and sludge conditioning of cationic polyacrylamide synthesized by a novel UVA-initiated system. Processes 2018, 6, 233. [Google Scholar] [CrossRef]
- Cai, T.; Li, H.J.; Yang, R.; Wang, Y.W. Efficient flocculation of an anionic dye from aqueous solutions using a cellulose-based flocculant. Cellulose 2015, 22, 1439–1449. [Google Scholar] [CrossRef]
- Ma, J.Y.; Shi, J.; Ding, H.C.; Zhu, G.C.; Fu, K.; Fu, X. Synthesis of cationic polyacrylamide by low-pressure UV initiation for turbidity water flocculation. Chem. Eng. J. 2017, 312, 20–29. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.W.; Cai, J.; Bai, J.F.; Yang, H.; Li, A.M. Preparation of dual-function starch-based flocculants for the simultaneous removal of turbidity and inhibition of E. coli in water. Water Res. 2016, 98, 128–137. [Google Scholar] [CrossRef]
- Bolto, B.; Gregory, J. Organic polyelectrolytes in water treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef]
- Lapointe, M.; Barbeau, B. Substituting polyacrylamide with an activated starch polymer during ballasted flocculation. J. Water Process Eng. 2019, 28, 129–134. [Google Scholar] [CrossRef]
- Kumar, K.; Adhikary, P.; Karmakar, N.C.; Gupta, S.; Singh, R.P.; Krishnamoorthi, S. Synthesis, characterization and application of novel cationic and amphoteric flocculants based on amylopectin. Carbohydr. Polym. 2015, 127, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.R.H.; Granville, A.M.; Browne, C.; Dagastine, R.R.; Yap, R.; Jefferson, B.; Henderson, R.K. Determining how polymer-bubble interactions impact algal separation using the novel “Posi”-dissolved air flotation process. Sep. Purif. Technol. 2018, 201, 139–147. [Google Scholar]
- Liu, F.; Ma, B.R.; Zhou, D.; Zhu, L.J.; Fu, Y.Y.; Xue, L.X. Positively charged loose nanofiltration membrane grafted by diallyl dimethyl ammonium chloride (DADMAC) via UV for salt and dye removal. React. Funct. Polym. 2015, 86, 191–198. [Google Scholar] [CrossRef]
- Lv, S.H.; Sun, T.; Zhou, Q.F.; Liu, J.J.; Ding, H.D. Synthesis of starch-g-p(DMDAAC) using HRP initiation and the correlation of its structure and sludge dewater ability. Carbohydr. Polym. 2014, 103, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Gao, B.Y.; Li, C.X.; Yue, Q.Y.; Liu, B. Synthesis and characterization of hydrophobically associating cationic polyacrylamide. Chem. Eng. J. 2010, 161, 27–33. [Google Scholar] [CrossRef]
- Wang, L.J.; Wang, J.P.; Yuan, S.J.; Zhang, S.J.; Tang, Y.; Yu, H.Q. Gamma radiation-induced dispersion polymerization in aqueous salts solution for manufacturing a cationic flocculant. Chem. Eng. J. 2009, 149, 118–122. [Google Scholar] [CrossRef]
- Mishra, S.; Mukul, A.; Sen, G.; Jha, U. Microwave assisted synthesis of polyacrylamide grafted starch (St-g-PAM) and its applicability as flocculant for water treatment. Int. J. Biol. Macromol. 2011, 48, 106–111. [Google Scholar] [CrossRef]
- Guan, Q.Q.; Zheng, H.L.; Zhai, J.; Zhao, C.; Zheng, X.K.; Tang, X.M.; Chen, W.; Sun, Y.J. Effect of template on structure and properties of cationic polyacrylamide: Characterization and mechanism. Ind. Eng. Chem. Res. 2014, 53, 5624–5635. [Google Scholar] [CrossRef]
- Zheng, H.L.; Ma, J.Y.; Zhu, C.J.; Zhang, Z.; Liu, L.W.; Sun, Y.J.; Tang, X.M. Synthesis of anion polyacrylamide under UV initiation and its application in removing dioctyl phthalate from water through flocculation process. Sep. Purif. Technol. 2014, 123, 35–44. [Google Scholar] [CrossRef]
- Ma, J.Y.; Fu, K.; Jiang, L.Y.; Ding, L.; Guan, Q.Q.; Zhang, S.H.; Zhang, H.W.; Shi, J.; Fu, X. Flocculation performance of cationic polyacrylamide with high cationic degree in humic acid synthetic water treatment and effect of kaolin particles. Sep. Purif. Technol. 2017, 181, 201–212. [Google Scholar] [CrossRef]
- Andreoli, F.C.; Sabogal-paz, L.P. Coagulation, flocculation, dissolved air flotation and filtration in the removal of Giardia spp. and Cryptosporidium spp. from water supply. Environ. Technol. 2019, 40, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, H.; Sun, Y.H.; Chiang, P.C.; Sun, W.Q.; Xu, Y.H.; Zheng, H.L.; Shah, K.J. Characterization and sludge dewatering performance evaluation of the photo-initiated cationic flocculant PDD. J. Taiwan Inst. Chem. Eng. 2018, 93, 253–262. [Google Scholar] [CrossRef]
- Sun, Q.; Zheng, H.L.; Zheng, X.Y.; Hu, X.B.; An, Y.Y.; Liu, H.X.; Zhao, C.L. Dual polydopamine-anion polyacrylamide polymer system for improved removal of nickel ions and methylene blue from aqueous solution. Sci. Adv. Mater. 2019, 11, 116–127. [Google Scholar] [CrossRef]
- Schwarz, D.; Weber, J. Synthesis of mesoporouspoly(melamine-formaldehyde) particles by inverse emulsion polymerization. J. Colloid Interface Sci. 2017, 498, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.L.; Zheng, H.L.; Gao, B.Y.; Liu, B.Z.; Zhai, J.; Zhang, S.X.; Xu, B.C. Ultrasound-initiated synthesis of cationic polyacrylamide for oily wastewater treatment: Enhanced interaction between the flocculant and contaminants. Ultrason. Sonochem. 2018, 42, 31–41. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Q.; Kong, F.; Fatehi, P. Production of cationic xylan–METAC copolymer as a flocculant for textile industry. Carbohydr. Polym. 2015, 124, 229–236. [Google Scholar] [CrossRef]
- Liao, Y.; Zheng, H.L.; Qian, L.; Sun, Y.J.; Dai, L.; Xue, W.W. Uv-initiated polymerization of hydrophobically associating cationic polyacrylamide modified by a surface-active monomer: A comparative study of synthesis, characterization, and sludge dewatering performance. Ind. Eng. Chem. Res. 2014, 53, 11193–11203. [Google Scholar] [CrossRef]
- Shaban, M.; S.A., A.R.; Ahadian, M.M.; Tamsilian, Y.; Weber, A.P. Facile synthesis of cauliflower-like hydrophobically modified polyacrylamide nanospheres by aerosol-photopolymerization. Eur. Polym. J. 2016, 83, 323–336. [Google Scholar] [CrossRef]
- Alange, V.V.; Birajdar, R.P.; Kulkarni, R.V. Functionally modified polyacrylamide-graft-gum karaya pH-sensitive spray dried microspheres for colon targeting of an anti-cancer drug. Int. J. Biol. Macromol. 2017, 102, 829–839. [Google Scholar] [CrossRef]
- Thapa, K.B.; Qi, Y.; Clayton, S.A.; Hoadley, A.F. Lignite aided dewatering of digested sewage sludge. Water Res. 2009, 43, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Huang, J.; Zeng, G.M.; Ruan, M.; Zhou, C.S.; Li, L.; Rong, Z.G. Optimization of flocculation conditions for kaolin suspension using the composite flocculant of MBFGA1 and PAC by response surface methodology. Bioresour. Technol. 2009, 100, 4233–4239. [Google Scholar] [CrossRef] [PubMed]
- Worasith, N.; Goodman, B.A.; Neampan, J.; Jeyachoke, N.; Thiravetyan, P. Characterization of modified kaolin from the Ranong deposit Thailand by XRD, XRF, SEM, FTIR and EPR techniques. Clay Miner. 2011, 46, 539–543. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, H.; Yang, H.; Li, H.B.; Li, A.M.; Cheng, R.S. Flocculation performance and mechanism of graphene oxide for removal of various contaminants from water. Water Res. 2013, 47, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Manafi, M.R.; Manafi, P.; Agarwal, S.; Bharti, A.K.; Asif, M.; Gupta, V.K. Synthesis of nanocomposites from polyacrylamide and graphene oxide: Application as flocculants for water purification. J. Colloid Interface Sci. 2017, 490, 505–510. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Fu, X.; Xia, W.; Fu, K.; Liao, Y. Flocculation of a High-Turbidity Kaolin Suspension Using Hydrophobic Modified Quaternary Ammonium Salt Polyacrylamide. Processes 2019, 7, 108. https://doi.org/10.3390/pr7020108
Ma J, Fu X, Xia W, Fu K, Liao Y. Flocculation of a High-Turbidity Kaolin Suspension Using Hydrophobic Modified Quaternary Ammonium Salt Polyacrylamide. Processes. 2019; 7(2):108. https://doi.org/10.3390/pr7020108
Chicago/Turabian StyleMa, Jiangya, Xue Fu, Wei Xia, Kun Fu, and Yi Liao. 2019. "Flocculation of a High-Turbidity Kaolin Suspension Using Hydrophobic Modified Quaternary Ammonium Salt Polyacrylamide" Processes 7, no. 2: 108. https://doi.org/10.3390/pr7020108