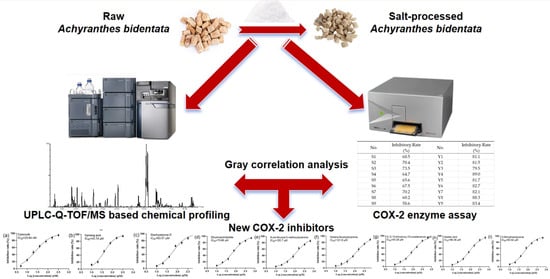
Optimizing the Salt-Processing Parameters of Achyranthes bidentata and Their Correlation with Anti-Osteoarthritis Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Chemicals
2.2. Optimization of Salt-Processing Procedure for A. bidentata
2.3. HPLC–Q-TOF-MS Analysis
2.4. COX-2 Inhibitory Assay
2.5. Gray Correlation Analysis
2.6. Chemometric Analysis
3. Results and Discussion
3.1. Optimization of the Salt-Processing Procedure for A. bidentata
3.2. Characterization of Chemical Constituents of Raw and Salt-Processed A. bidentata
3.3. Chemometric Analysis
3.4. Gray Correlation Analysis
3.5. COX-2 Inhibitory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, P.; Tan, S.; Zhang, Y.X.; Li, J.S.; Chai, C.; Li, J.J.; Cai, B.C. The effects of wine-processing on ascending and descending: The distribution of flavonoids in rat tissues after oral administration of crude and wine-processed Radix scutellariae. J. Ethnopharmacol. 2014, 155, 649–664. [Google Scholar] [CrossRef]
- Tao, Y.; Du, Y.; Li, W.; Cai, B.; Di, L.; Shi, L.; Hu, L. Integrating UHPLC-MS/MS quantification and DAS analysis to investigate the effects of wine-processing on the tissue distributions of bioactive constituents of herbs in rats: Exemplarily shown for Dipsacus asper. J. Chromatogr. B 2017, 1055–1056, 135–143. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, X.; Wang, X.; Cao, G.; Qin, K.; Pei, K.; Zhu, H.; Cai, H.; Niu, M.; Cai, B. Profiling and analysis of multiple compounds in rhubarb decoction after processing by wine steaming using UHPLC-Q-TOF-MS coupled with multiple statistical strategies. J. Sep. Sci. 2016, 39, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ren, C.; Fei, C.; Wang, Y.; Xue, Q.; Li, L.; Yin, F.; Li, W. Analysis of the chemical composition changes of Gardeniae Fructus before and after processing based on ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2021, 44, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Wang, B.; Li, W.; Cai, H.; Chen, D.; Liu, X.; Yin, F.; Cai, B. Quality assessment of raw and processed Arctium lappa L. through multicomponent quantification, chromatographic fingerprint, and related chemometric analysis. J. Sep. Sci. 2015, 38, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X. The genus Achyranthes: A review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2017, 203, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, D.; Peng, L.; Li, Y.; Liao, Z.; Yu, T. Compatibility of Achyranthes bidentata components in reducing inflammatory response through Arachidonic acid pathway for treatment of Osteoarthritis. Bioengineered 2022, 13, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Gawande, D.Y.; Goel, R.K. Pharmacological validation of in-silico guided novel nootropic potential of Achyranthes aspera L. J. Ethnopharmacol. 2015, 175, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, A.; Muhammad, I.; Ullah, B.; Muhammad, N.; Muhammad, Z.; Ali, N. Pharmacognostic and hypoglycemic studies of Achyranthus aspera L. J. Pharmacognosy Phytother. 2013, 5, 127–131. [Google Scholar] [CrossRef]
- Pan, R.; Hu, W.; Pan, J.; Huang, L.; Luan, C.; Shen, H. Achyranthes bidentata polypeptides prevent apoptosis by inhibiting the glutamate current in cultured hippocampal neurons. Neural Regen. Res. 2020, 15, 1086–1093. [Google Scholar]
- Nazir, A.; Saleem, M.A.; Nazir, F.; Hussain, T.; Faizan, M.Q.; Usman, M. Comparison of UV Protection Properties of Cotton Fabrics Treated with Aqueous and Methanolic Extracts of Achyranthes aspera and Alhagi maurorum Plants. Photochem. Photobiol. 2016, 92, 343–347. [Google Scholar] [CrossRef]
- Gawande, D.Y.; Druzhilovsky, D.; Gupta, R.C.; Poroikov, V.; Goel, R.K. Anticonvulsant activity and acute neurotoxic profile of Achyranthes aspera Linn. J. Ethnopharmacol. 2017, 202, 97–102. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Zhang, D.; Wang, C.; Yan, C. Anti-osteoporosis activity of a novel Achyranthes bidentata polysaccharide via stimulating bone formation. Carbohyd. Polym. 2018, 184, 288–298. [Google Scholar] [CrossRef]
- Lee, T.G.; Hyun, S.W.; Jo, K.; Park, B.; Lee, I.S.; Song, S.J.; Kim, C.S. Achyranthis radix Extract Improves Urban Particulate Matter-Induced Dry Eye Disease. Int. J. Environ. Res. Public Health 2019, 16, 3229. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, M.; Li, B.; Kan, Y.; Zhang, B.; Zheng, X.; Feng, W. Raw and salt-processed Achyranthes bidentata attenuate LPS-induced acute kidney injury by inhibiting ROS and apoptosis via an estrogen-like pathway. Biomed. Pharmacother. 2020, 129, 110403. [Google Scholar] [CrossRef]
- Nunavath, R.S.; Singh, M.T.; Jain, A.; Chakma, M.; Arivuselvam, R.; Azeeze, M. Quality by Design in Pharmaceuticals: A Review of its Impact on Regulatory Compliance and Product Quality. Drug Res. 2024, 74, 18–23. [Google Scholar]
- Abramson, S.B. The role of COX-2 produced by cartilage in arthritis. Osteoarthr. Cartil. 1999, 7, 380–381. [Google Scholar] [CrossRef] [PubMed]
- El-Miligy MM, M.; Al-Kubeisi, A.K.; El-Zemity, S.R.; Nassra, R.A.; Abu-Serie, M.M.; Hazzaa, A.A. Discovery of small molecule acting as multitarget inhibitor of colorectal cancer by simultaneous blocking of the key COX-2, 5-LOX and PIM-1 kinase enzymes. Bioorg. Chem. 2021, 115, 105171. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wu, H.; Hong, Z.; Wei, F.; Zhao, M.; Tang, R.; Li, Y.; Ge, W.; Li, C.; Du, W. Exploring active ingredients of anti-osteoarthritis in raw and wine-processed Dipsaci Radix based on spectrum-effect relationship combined with chemometrics. J. Ethnopharmacol. 2023, 309, 116281. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Y.; Gao, Y.; Yu, X.; Zhao, C.; Song, X.; Han, F.; Yu, J. Spectrum-effect relationship analysis based on HPLC-FT-ICR-MS and multivariate statistical analysis to reveal the pharmacodynamic substances of Ling-Gui-Zhu-Gan decoction on Alzheimer’s disease. J. Pharm. Biomed. Anal. 2024, 237, 115765. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Siddiqui, N.A.; Rehman, M.T.; Hussain, A.; Akhtar, A.; Mir, S.R.; Alajmi, M.F. Box–Behnken Design (BBD)-Based Optimization of Microwave-Assisted Extraction of Parthenolide from the Stems of Tarconanthus camphoratus and Cytotoxic Analysis. Molecules 2021, 26, 1876. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Tu, X.; Zang, Q.; Zhu, Y.; Li, L.; Zhang, R.; Abliz, Z. Liquid chromatography-mass spectrometry-based metabolomics and fluxomics reveals the metabolic alterations in glioma U87MG multicellular tumor spheroids versus two-dimensional cell cultures. Rapid Commun. Mass Spectrom. 2024, 38, e9670. [Google Scholar] [CrossRef]
- Sun, J.; Song, Y.; Sun, H.; Liu, W.; Zhang, Y.; Zheng, J.; Zhang, Q.; Zhao, Y.; Xiao, W.; Tu, P.; et al. Characterization and quantitative analysis of phenolic derivatives in Longxuetongluo Capsule by HPLC-DAD-IT-TOF-MS. J. Pharm. Biomed. Anal. 2017, 145, 462–472. [Google Scholar] [CrossRef]
- Yao, C.; Wang, Y.; Qu, H.; Li, J.; Hou, J.; Chen, X.; Zhang, J.; Wei, W.; Bi, Q.; Guo, D.A. Comparative identification of phytoecdysteroids in Achyranthes bidentata Blume and its three analogous species and application in differentiation between processing products from different species. J. Pharm. Biomed. Anal. 2023, 227, 115187. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.Q.; Luo, Y.; Shang, J.C.; Jiang, X.H. Simultaneous determination of eleven compounds related to metabolism of bioamines in rat cortex and hippocampus by HPLC-ECD with boron-doped diamond working electrode. J. Pharm. Biomed. Anal. 2016, 118, 41–51. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, S.; Dabur, R. Higher plants exert interspecific effects on the phytoecdysteroids contents in Tinospora cordifolia. Chem. Bio. Lett. 2022, 9, 312. [Google Scholar]
- Bourne, P.C.; Whiting, P.; Dhadialla, T.S.; Hormann, R.E.; Girault, J.P.; Harmatha, J.; Lafont, R.; Dinan, L. Ecdysteroid 7,9(11)-dien-6-ones as potential photoaffinity labels for ecdysteroid binding proteins. J. Insect Sci. 2002, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, H.; Wang, Q.H.; Yang, B.Y.; Kuang, H.X. A new feruloyl tyramine glycoside from the roots of Achyranthes bidentata. Chin. J. Nat. Med. 2012, 10, 16–19. [Google Scholar] [CrossRef]
- Souza, L.; Oliveira, J.; Fernandes AD, S.; Macedo, A.F.; Araujo-Lima, C.F.; Felzenszwalb, I. UHPLC-MS metabolomic profile and in silico pharmacokinetic approach of Kalanchoe daigremontiana Raym.-Hamet & H. Perrier aqueous extracts. J. Pharm. Biomed. Anal. 2024, 238, 115827. [Google Scholar]
- Hamberg, M.; Olsson, U. Efficient and specific conversion of 9-lipoxygenase hydroperoxides in the beetroot. Formation of pinellic acid. Lipids 2011, 46, 873–878. [Google Scholar] [CrossRef]
- Fuchs, D.; Tang, X.; Johnsson, A.K.; Dahlén, S.E.; Hamberg, M.; Wheelock, C.E. Eosinophils synthesize trihydroxyoctadecenoic acids (TriHOMEs) via a 15-lipoxygenase dependent process. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158611. [Google Scholar] [CrossRef]
- Khan, R.S.; Senthi, M.; Rao, P.C.; Basha, A.; Alvala, M.; Tummuri, D.; Masubuti, H.; Fujimoto, Y.; Begum, A.S. Cytotoxic constituents of Abutilon indicum leaves against U87MG human glioblastoma cells. Nat. Prod. Res. 2015, 29, 1069–1073. [Google Scholar] [CrossRef]
- Mei, Y.; Zhang, X.; Hu, Y.; Tong, X.; Liu, W.; Chen, X.; Cao, L.; Wang, Z.; Xiao, W. Screening and characterization of xenobiotics in rat bio-samples after oral administration of Shen-Wu-Yi-Shen tablet using UPLC-Q-TOF-MS/MS combined with a targeted and non-targeted strategy. J. Pharm. Biomed. Anal. 2023, 227, 115286. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, P.G. Simultaneous determination of multi-class active pharmaceutical ingredients by UHPLC-HRMS. J. Pharm. Biomed. Anal. 2021, 202, 114160. [Google Scholar] [CrossRef]
- Aly, S.H.; Elissawy, A.M.; Mahmoud, A.M.A.; El-Tokhy, F.S.; Mageed, S.S.A.; Almahli, H.; Al-Rashood, S.T.; Binjubair, F.A.; Hassab, M.A.E.; Eldehna, W.M.; et al. Synergistic Effect of Sophora japonica and Glycyrrhiza glabra Flavonoid-Rich Fractions on Wound Healing: In Vivo and Molecular Docking Studies. Molecules 2023, 28, 2294. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, L.; Xu, L.; Zhang, J.; Li, J.; Gao, M.; Yu, J.; Jin, L.; Lei, D. UHPLC-QTOF-MS/MS based characterization of anti-tumor constituents in Ceratocarpus arenarius L. and identification of EGFR-TK inhibitors by virtual screening. Nat. Prod. Res. 2022, 36, 6111–6115. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Lan, L.; Gong, D.; Guo, Y.; Sun, G. Evaluation of quality consistency of herbal preparations using five-wavelength fusion HPLC fingerprint combined with ATR-FT-IR spectral quantized fingerprint: Belamcandae rhizoma antiviral injection as an example. J. Pharm. Biomed. Anal. 2022, 214, 114733. [Google Scholar] [CrossRef] [PubMed]
- Yi-meng, Y.; Ke-xin, Y.; Yu-sheng, L.; Ge, C.; Han-wen, T.; Zhongying, L.; Zhiqiang, L.; Fengrui, S.; Zifeng, P. Characterization of Components in Huangying Kechuan Syrup by Ultra-high Liquid Chromatography Tandem Quadrupole-time-of-flight Mass Spectrometry. Chin. J. App. Chem. 2021, 38, 276. [Google Scholar]
- Fadaly, W.A.A.; Nemr, M.T.M.; Zidan, T.H.; Mohamed, F.E.A.; Abdelhakeem, M.M.; Abu Jayab, N.N.; Omar, H.A.; Abdellatif, K.R.A. New 1,2,3-triazole/1,2,4-triazole hybrids linked to oxime moiety as nitric oxide donor selective COX-2, aromatase, B-RAF(V600E) and EGFR inhibitors celecoxib analogs: Design, synthesis, anti-inflammatory/anti-proliferative activities, apoptosis and molecular modeling study. J. Enzym. Inhib. Med. Chem. 2023, 38, 2290461. [Google Scholar]
- Jiang, Y.; Yu, L.; Wang, M.H. N-trans-feruloyltyramine inhibits LPS-induced NO and PGE2 production in RAW 264.7 macrophages: Involvement of AP-1 and MAP kinase signalling pathways. Chem. Biol. Interact. 2015, 235, 56–62. [Google Scholar] [CrossRef] [PubMed]






| Run | Independent Variable | Conc. of Ecdysterone (μg/mL) | ||
|---|---|---|---|---|
| X1 (Soak Time, min) | X2 (Concentration of Brine, %) | X3 (Stir-Frying Time, min) | ||
| 1 | 30 | 2 | 5 | 485.571 |
| 2 | 30 | 1 | 7 | 305.3 |
| 3 | 10 | 2 | 7 | 395.933 |
| 4 | 30 | 3 | 7 | 395.356 |
| 5 | 50 | 2 | 7 | 391.509 |
| 6 | 50 | 3 | 5 | 389.812 |
| 7 | 30 | 2 | 5 | 488.776 |
| 8 | 10 | 3 | 5 | 421.638 |
| 9 | 30 | 2 | 5 | 484.89 |
| 10 | 10 | 2 | 3 | 410.132 |
| 11 | 30 | 2 | 5 | 486.46 |
| 12 | 30 | 1 | 3 | 446.888 |
| 13 | 10 | 1 | 5 | 436.884 |
| 14 | 50 | 2 | 3 | 457.694 |
| 15 | 30 | 2 | 5 | 480.762 |
| 16 | 50 | 1 | 5 | 438.679 |
| 17 | 30 | 3 | 3 | 463.877 |
| Factor | Regression Coefficients | Standard Error | DF | F Value | Prob > F |
|---|---|---|---|---|---|
| B0 | 485.29 | 13.60 | 1 | 3.89 | 0.0435 |
| Linear | |||||
| B1 | 1.64 | 10.75 | 1 | 0.0232 | 0.8831 |
| B2 | 5.37 | 10.75 | 1 | 0.2493 | 0.6329 |
| B3 | −36.31 | 10.75 | 1 | 11.41 | 0.0118 |
| Interaction | |||||
| β12 | −8.41 | 15.20 | 1 | 0.3058 | 0.5975 |
| β13 | −13.0 | 15.20 | 1 | 0.7311 | 0.4208 |
| β23 | 18.27 | 15.20 | 1 | 1.44 | 0.2685 |
| Quadratic | |||||
| β11 | −26.29 | 14.81 | 1 | 3.15 | 0.1193 |
| β22 | −37.25 | 14.81 | 1 | 6.32 | 0.0401 |
| β33 | −45.19 | 14.81 | 1 | 9.30 | 0.0186 |
| Lack of fit | 3 | 250.38 | <0.0001 | ||
| Pure error | 4 | ||||
| R2 | 0.8334 | Adjusted R2 | 0.6192 |
| Factor | Value |
|---|---|
| Soak time (min) | 29 |
| Concentration of brine (%) | 1.8 |
| Stir-frying time (min) | 4.4 |
| Predicted values (μg/mL) | 490.1 |
| Experimental values (μg/mL) | 491.79 ± 0.83 |
| No. | tR (min) | Detected Mass (m/z) | MS2 | Molecular Formula | Mass Error (ppm) | Compound (Name) | Identification | Peak Area Ratio |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.50 | 115.0036 | 100.0234, 99.0085, 99.9332 | C4H4O4 | 4.3 | Maleic acid | standards | 1.91 |
| 2 | 2.81 | 115.0032 | 111.1115, 104.7421, 87.0104, 77.8366, 73.9177 | C4H4O4 | 0.9 | Unknown | — | 26.59 |
| 3 | 3.34 | 115.0037 | 111.0400, 108.0446, 105.0223, 97.0370, 89.0271, 87.0083, 59.0153 | C4H4O4 | 5.2 | Fumaric acid | standards | 2.04 |
| 4 | 4.47 | 204.0664 | 186.0639, 159.0353, 131.0420, 108.0486, 69.8676 | C11H11NO3 | 1.5 | Indolyllactic acid | [22] | 12.55 |
| 5 | 5.1 | 235.0757 | 220.0741, 187.0871, 141.6590, 122.0250, 94.0303, 93.0204, 77.4244 | C16H12O2 | −0.9 | Methylflavone | [23] | 6.97 |
| 6 | 6.07 | 541.3029 | 495.2991, 477.2920, 299.1640, 249.1525, 129.0598, 99.0488 | C27H44O8 | 3.0 | Polypodine B | [24] | 4.93 |
| 7 | 7.32 | 210.0773 | 177.0686, 154.0703, 124.0408, 122.0292, 94.0312, 93.0255, 66.0354 | C10H13NO4 | 3.3 | Methoxytyrosine | [25] | 10.63 |
| 8 | 8.43 | 525.3055 | 479.3034, 319.1958, 301.1819, 210.0828, 159.1058 | C27H44O7 | −1.7 | Ecdysterone | standards | 0.80 |
| 9 | 9.18 | 525.3040 | 479.3073, 443.1053, 377.1133, 346.1055, 319.1931, 301.1936, 282.1422, 261.1450, 217.1015, 186.0648, 159.1079, 141.0920, 124.0425 | C27H44O7 | −4.6 | 25R-inokosterone | standards | 0.90 |
| 10 | 9.51 | 525.3048 | 479.3033, 477.2902, 159.1055 | C27H44O7 | −3.0 | 25S-inokosterone | standards | 0.80 |
| 11 | 13.4 | 507.297 | 461.2921, 301.1889, 277.1472, 249.1255, 201.1278, 159.1051 | C27H42O6 | 2.4 | Dehydroecdysone | standards | 7.28 |
| 12 | 14.7 | 507.296 | 461.2861, 345.1842, 309.1479, 265.1693, 229.0488, 159.1066, 115.0768, 83.0535 | C27H42O6 | 1.0 | Deoxykaladasterone | [26] | 19.43 |
| 13 | 15.7 | 523.3109 | 477.3076, 459.2956, 361.2192, 317.1931, 159.1105 | C27H42O7 | −1.5 | Kaladasterone | [27] | 15.25 |
| 14 | 16.4 | 507.2959 | 461.2989, 403.2529, 301.1815, 249.1308, 209.1000, 159.1058, 83.0489 | C27H42O6 | 0.2 | Dacryhainansterone | [26] | 0.58 |
| 15 | 17.5 | 187.0974 | 159.1022, 141.1016, 139.0442, 111.0111 | C9H16O4 | 2.1 | Azelaic acid | standards | 4.38 |
| 16 | 18.6 | 549.1589 | 341.1035, 311.0556, 295.0618, 268.0379, 255.0433, 252.0448 | C26H30O13 | −4.4 | Liquiritin apioside | standards | 1.38 |
| 17 | 21.5 | 342.1357 | 327.1139, 190.0521, 178.0531, 148.0552, 135.0417, 134.0422 | C19H21NO5 | 4.7 | N-cis-feruloyl-3-methoxytyramine | standards | 0.26 |
| 18 | 24.4 | 312.1246 | 190.0584, 178.0544, 148.0556, 135.0491 | C18H19NO4 | 3.2 | N-trans-feruloyltyramine | standards | 0.81 |
| 19 | 25.4 | 342.1343 | 327.1171, 190.0533, 178.0529, 148.0544, 135.0460 | C19H21NO5 | 1.2 | Feruloylmethoxytyramine | [28] | 0.91 |
| 20 | 29.0 | 509.2856 | 494.1100, 489.6156, 466.2194, 421.8323, 410.1779, 387.1925, 384.8494, 358.5213, 350.2032, 342.0960, 333.4114 | C32H46O5 | −1.8 | Unknown | — | 2.26 |
| 21 | 30.5 | 225.1124 | 214.0546, 209.0778, 196.8297, 187.0986, 171.8831, 158, 1576, 144.2472, 130.0387, 116.0739, 98.9125, 96.5702 | C11H16O2 | −1.3 | Dihydroactinidiolide | standards | 0.65 |
| 22 | 32.1 | 507.2973 | 461.2921, 368.0941, 342.1358, 312.1195, 268.0684, 242.0818, 221.7878, 171.0949, 139.1091, 86.8855, 56.2580 | C27H42O6 | 4.9 | Stachysterone D | standards | 10.49 |
| 23 | 32.9 | 327.2175 | 229.1391, 221.1249, 211.1358, 171.1032, 85.0286 | C18H32O5 | 1.2 | Corchorifatty acid F | [29] | 0.87 |
| 24 | 33.4 | 327.2186 | 323.1074, 242.4109, 235.1030, 211.1431, 171.1025, 146.9710, 137.1052, 97.0710, 85.0290 | C18H32O5 | 4.6 | 9,12,13-Trihydroxy-10,15-octadecadienoic acid | [30] | 0.93 |
| 25 | 35.8 | 329.2340 | 229.1482, 211.1358, 171.1053, 139.1160 | C18H34O5 | 3.6 | 9,12,13-Trihydroxy-10-octadecenoic acid | standards | 0.51 |
| 26 | 36.3 | 329.2337 | 171.1043, 139.1148, 127.1147 | C18H34O5 | 2.7 | 9,10,13-TriHOME | [31] | 0.61 |
| 27 | 39.3 | 329.2334 | 211.2371, 199.1361, 197.1192, 181.1294, 169.1259, 129.0907, 99.0819 | C18H34O5 | 1.8 | Pinellic acid | [32] | 0.71 |
| 28 | 43.2 | 329.2336 | 303.0145, 255.2305, 204.8009, 211.1199, 201.1148, 199.1152, 171.1017, 152.9974, 146.9705, 96.9641, 80.8853 | C18H34O5 | 2.4 | Sanleng acid | standards | 0.55 |
| 29 | 53.2 | 643.3685 | 610.5566, 588.5043, 569.3351, 531.3790, 512.9322 | C36H54O10 | 7.1 | Unknown | — | 0.80 |
| No. | Inhibitory Rate (%) | No. | Inhibitory Rate (%) |
|---|---|---|---|
| S1 | 68.5 | Y1 | 81.1 |
| S2 | 70.4 | Y2 | 81.5 |
| S3 | 73.5 | Y3 | 79.5 |
| S4 | 64.7 | Y4 | 89.0 |
| S5 | 65.6 | Y5 | 81.7 |
| S6 | 67.5 | Y6 | 82.7 |
| S7 | 70.2 | Y7 | 82.1 |
| S8 | 60.2 | Y8 | 88.3 |
| S9 | 58.6 | Y9 | 83.4 |
| No. | Compounds | Correlation Coefficient r |
|---|---|---|
| 1 | Maleic acid | 0.7450 |
| 2 | Unknown | 0.7574 |
| 3 | Fumaric acid | 0.7326 |
| 4 | Indolyllactic acid | 0.8394 |
| 5 | Methylflavone | 0.8060 |
| 6 | Polypodine B | 0.8358 |
| 7 | Methoxytyrosine | 0.7346 |
| 8 | Ecdysterone | 0.8378 |
| 9 | 25R-inokosterone | 0.7544 |
| 10 | 25S-inokosterone | 0.8381 |
| 11 | Dehydroecdysone | 0.8506 |
| 12 | Deoxykaladasterone | 0.7291 |
| 13 | Kaladasterone | 0.7156 |
| 14 | Dacryhainansterone | 0.7267 |
| 15 | Azelaic acid | 0.8925 |
| 16 | Liquiritin apioside | 0.7818 |
| 17 | N-cis-feruloyl-3-methoxytyramine | 0.9175 |
| 18 | N-trans-feruloyltyramine | 0.9118 |
| 19 | Feruloylmethoxytyramine | 0.8186 |
| 20 | Unknown | 0.6732 |
| 21 | Dihydroactinidiolide | 0.9186 |
| 22 | Stachysterone D | 0.9291 |
| 23 | Corchorifatty acid F | 0.7514 |
| 24 | 9,12,13-Trihydroxy-10,15-octadecadienoic acid | 0.8251 |
| 25 | 9,12,13-Trihydroxy-10-octadecenoic acid | 0.9107 |
| 26 | 9,10,13-triHOME | 0.7601 |
| 27 | Pinellic acid | 0.6485 |
| 28 | Sanleng acid | 0.9981 |
| 29 | Unknown | 0.7320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Shen, L.; Shen, G.; Tao, Y. Optimizing the Salt-Processing Parameters of Achyranthes bidentata and Their Correlation with Anti-Osteoarthritis Effect. Processes 2024, 12, 434. https://doi.org/10.3390/pr12030434
Zhu J, Shen L, Shen G, Tao Y. Optimizing the Salt-Processing Parameters of Achyranthes bidentata and Their Correlation with Anti-Osteoarthritis Effect. Processes. 2024; 12(3):434. https://doi.org/10.3390/pr12030434
Chicago/Turabian StyleZhu, Jieqiang, Lisha Shen, Guofang Shen, and Yi Tao. 2024. "Optimizing the Salt-Processing Parameters of Achyranthes bidentata and Their Correlation with Anti-Osteoarthritis Effect" Processes 12, no. 3: 434. https://doi.org/10.3390/pr12030434